General Discussion
Related: Editorials & Other Articles, Issue Forums, Alliance Forums, Region ForumsDU science/weather buffs: Is sea water evaporate turned into fresh rain water, and how?
htuttle
(23,738 posts)When water evaporates, the salt stays 'on the ground', while the water vapor floats away.
For the more sciencey among us, that's because salt water is a solution, not a compound.
MarvinGardens
(779 posts)And, for those who want to do a little science in their kitchen: heat a little salt water in a pot with a lid. Probably won't need to come to a full boil, just enough to get some condensation on the underside of the lid. Then carefully collect some of the condensate in a saucer or similar, without burning yourself. When cool enough, taste the condensate. Is it salty?
jberryhill
(62,444 posts)I was writing up the procedure below while you posted that
brush
(53,735 posts)iscooterliberally
(2,859 posts)The answer is that the Sun puts energy onto the Earth's surface and that includes the ocean surface, and every square metre gets energy at the rate of, on average, about 1 kilowatt, so 1,000 joules per second. This gives energy to the particles of water in the sea and dissolved in the sea are obviously some ions, sodium and chloride, and lots of others as well, that's why the sea is salty. But the water molecules, although they are sticky and they stick to other water molecules because they are what's called dipole molecules, when you give enough energy to the water molecule, it can break the bonds to the other water molecules, holding it into the water, and it can escape as water vapour.
It isn't possible to give energy in the same way to the ions, or at least not sufficient energy to make them boil off and get into a vapour state like the water. And this is because they are charged and that charge on them makes them a lot stickier, and they interact with other molecules of water, and other ions in the water, far more strongly than a water molecule does. So, it's far easier for water to escape at the temperatures that we see than those other ion species.
more at the link below.
https://www.thenakedscientists.com/articles/questions/why-isnt-rain-salty
brush
(53,735 posts)hlthe2b
(102,106 posts)bigbrother05
(5,995 posts)The increasing intensity of hurricanes and rain events tracks closely with the higher sea temperatures.
There is more energy in the system along with more moisture to act on.
jberryhill
(62,444 posts)This will take you a few minutes:
1. Put a plate in your refrigerator.
2. Put some water in a tea kettle or pot.
3. Dissolve a bunch of salt into the water in the kettle or pot.
4. Put the pot on the stove and heat to boiling.
5. Remove the plate from your refrigerator and hold it in the steam coming off the kettle or pot, until it is good and wet from the steam condensing on it.
6. Set the plate aside to cool.
7. Lick the plate.
Does the water on the plate taste salty?
(a) Yes
(b) No
Now, you could say, "But the water in the ocean isn't boiling."
Well, yes it is, it's just doing it very slowly.
At ordinary temperatures in which you and water find yourselves, the water exists in equilibrium with water vapor above it. Water molecules in the water have a distribution of kinetic energy, centered around an average kinetic energy level which determines the temperature of the water. Some of the more energetic ones are constantly escaping from the surface and becoming water vapor. This happens across the temperature range. But it happens a lot more often when the water is hot, so you are just speeding up the process of evaporation.
Now, when you are talking about an ocean, warming increases the rate of evaporation. As the water vapor rises, it takes on a vortex movement due to the earth's rotation (the Coriolis Effect), and becomes a hurricane.
Lochloosa
(16,057 posts)jberryhill
(62,444 posts)sdfernando
(4,923 posts)I used to work it a lot when I was younger...anyway, we had soft-water to wash and rinse the cars. In the pump room were big vats that held salt to filter the impurities of the notoriously hard San Diego water. We bought that salt from a local company. They had a facility at the end of the bay. They flooded their area with bay water (sea water) then sealed off form the bay. Let nature take its course to evaporate the water and then harvested the salt left behind. So I could see visually that salt doesn't evaporate.
brush
(53,735 posts)jberryhill
(62,444 posts)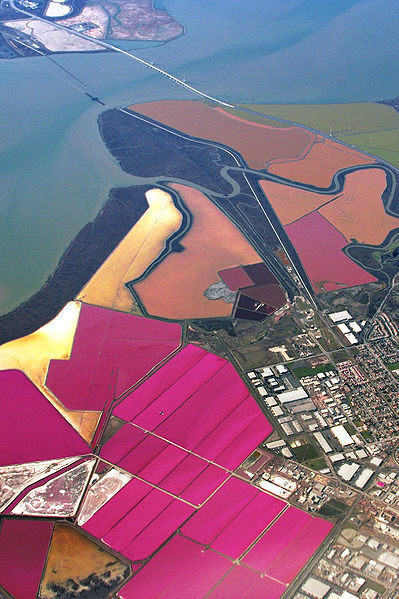
Other pictures at link.
There's a raft of these around the Great Salt Lake in Utah as well.
The reason, of course, for things like the Great Salt Lake, the Bonneville Salt Flats, the Dead Sea, etc. is that they are arid low-lying areas into which water drains down from the mountains picking up mineral salts along the way, evaporates, and leaves the salts behind. That is how the oceans became salty in the first place is by eons of fresh water flowing into them, evaporating, and leaving the minerals behind.
This is Bonneville:

Death Valley:
