Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Environment & Energy
Related: About this forumColumbia Engineers Develop New, Low-Cost Way to Capture Carbon
http://engineering.columbia.edu/columbia-engineers-develop-new-low-cost-way-capture-carbon[font face=Serif][font size=5]Columbia Engineers Develop New, Low-Cost Way to Capture Carbon[/font]
Jun. 23, 2016
[font size=3]A recent study led by Xi Chen, associate professor of earth and environmental engineering at Columbia Engineering, and Klaus Lackner at Arizona State University, reports an unconventional reversible chemical reaction in a confined nanoenvironment. The discovery, a milestone in clarifying the scientific underpinnings of moisture-swing chemical reaction, is critical to understanding how to scrub CO₂ from the Earth's atmosphere, and the researchers have already used it to capture CO₂ more efficiently and at a much lower cost than other methods.
[center][font size=1]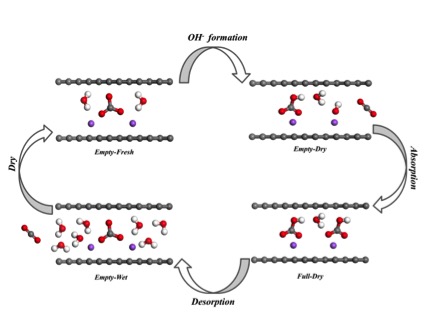
Reaction pathway of CO₂ absorbption/desorption on nanostructural absorbent[/font][/center]
Water is the key player in this new study. The group found that reducing water quantities in nanoconfinement could promote CO₃²- (carbonate) ions to hydrolyze H₂O into a larger amount of OH- (hydroxide) ions. This discovery also led the team to find a new nanostructured CO₂ sorbent (a material used to absorb or adsorb liquids or gases) that also binds CO₂ spontaneously in ambient air when the surrounding is dry, while releasing it when exposed to moisture. The work was published in Angewandte Chemie in February 2016.
“Water confined in nanoscopic pores is essential in determining the energetics of many chemical, physical, biological, and environmental systems,” says Chen. “Our finding sheds light on a vast number of chemical processes in nanoconfinement while also giving rise to a wide array of potential applications. For instance, we can convert this new efficient sorbent from absorption to desorption simply by using water, which is readily available and at very low-cost. Current sorbent materials consume a great deal of energy, so our discovery could lead to cheaper and more efficient energy conservation absorbents. And if we can achieve negative carbon emission standards, then we will have invented a nanomaterial solution to a critical global challenge.”
Finding an efficient absorbent has long been a challenge for most absorption and desorption processes. A successful CO₂ absorbent must have fast reaction kinetics (the rate of chemical processes), be low in cost, and be able to regenerate with a low energy barrier to complete the whole CO₂ capture-release cycle. Chen notes that, to his knowledge, all previous CO₂ absorbents have required a large energy barrier to regenerate and are thus not very efficient, consuming more extra energy to regenerate. The mechanism of the moisture-swing chemical reaction in nanopores will lead to new classes of sorbents driven by water: evaporation in ambient air through solar energy drives the sorbent to absorb CO₂ as it dries, and hydration releases CO₂ when wet.
…[/font][/font]
Jun. 23, 2016
[font size=3]A recent study led by Xi Chen, associate professor of earth and environmental engineering at Columbia Engineering, and Klaus Lackner at Arizona State University, reports an unconventional reversible chemical reaction in a confined nanoenvironment. The discovery, a milestone in clarifying the scientific underpinnings of moisture-swing chemical reaction, is critical to understanding how to scrub CO₂ from the Earth's atmosphere, and the researchers have already used it to capture CO₂ more efficiently and at a much lower cost than other methods.
[center][font size=1]

Reaction pathway of CO₂ absorbption/desorption on nanostructural absorbent[/font][/center]
Water is the key player in this new study. The group found that reducing water quantities in nanoconfinement could promote CO₃²- (carbonate) ions to hydrolyze H₂O into a larger amount of OH- (hydroxide) ions. This discovery also led the team to find a new nanostructured CO₂ sorbent (a material used to absorb or adsorb liquids or gases) that also binds CO₂ spontaneously in ambient air when the surrounding is dry, while releasing it when exposed to moisture. The work was published in Angewandte Chemie in February 2016.
“Water confined in nanoscopic pores is essential in determining the energetics of many chemical, physical, biological, and environmental systems,” says Chen. “Our finding sheds light on a vast number of chemical processes in nanoconfinement while also giving rise to a wide array of potential applications. For instance, we can convert this new efficient sorbent from absorption to desorption simply by using water, which is readily available and at very low-cost. Current sorbent materials consume a great deal of energy, so our discovery could lead to cheaper and more efficient energy conservation absorbents. And if we can achieve negative carbon emission standards, then we will have invented a nanomaterial solution to a critical global challenge.”
Finding an efficient absorbent has long been a challenge for most absorption and desorption processes. A successful CO₂ absorbent must have fast reaction kinetics (the rate of chemical processes), be low in cost, and be able to regenerate with a low energy barrier to complete the whole CO₂ capture-release cycle. Chen notes that, to his knowledge, all previous CO₂ absorbents have required a large energy barrier to regenerate and are thus not very efficient, consuming more extra energy to regenerate. The mechanism of the moisture-swing chemical reaction in nanopores will lead to new classes of sorbents driven by water: evaporation in ambient air through solar energy drives the sorbent to absorb CO₂ as it dries, and hydration releases CO₂ when wet.
…[/font][/font]
InfoView thread info, including edit history
TrashPut this thread in your Trash Can (My DU » Trash Can)
BookmarkAdd this thread to your Bookmarks (My DU » Bookmarks)
1 replies, 588 views
ShareGet links to this post and/or share on social media
AlertAlert this post for a rule violation
PowersThere are no powers you can use on this post
EditCannot edit other people's posts
ReplyReply to this post
EditCannot edit other people's posts
Rec (2)
ReplyReply to this post
1 replies
 = new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
= new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
Columbia Engineers Develop New, Low-Cost Way to Capture Carbon (Original Post)
OKIsItJustMe
Jun 2016
OP
tonyt53
(5,737 posts)1. Good stuff!