Environment & Energy
Related: About this forumNew Economic Water-Splitting Catalyst, Ru@C₂N
http://news.unist.ac.kr/new-economic-water-splitting-catalyst-ruc%e2%82%82n/[font size=4]Their findings published in the recent issue of the journal Nature Nanotechnology.[/font]
Feb 14, 2017 | Joo Hyeon Heo | Public Relations Team
[font size=3]UNIST scientists have developed an exiting new catalyst that can split water into hydrogen almost as good as platinum, but less costly and found frequently on Earth.
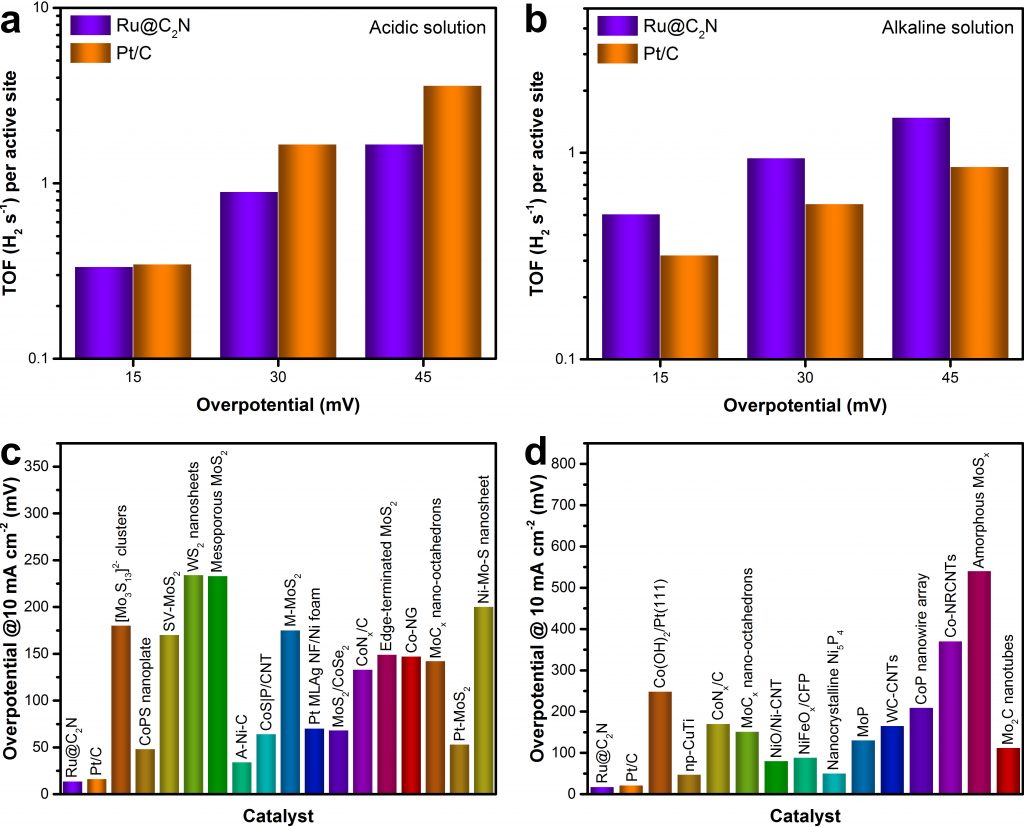
As described in the journal Nature Nanotechnology, this ruthenium (Ru)-based material works almost as efficient as platinum and likely shows the highest catalytic performance without being affected by the pH of the water.
The research team, led by Professor Jong-Beom Baek of the Energy and Chemical Engineering at UNIST has synthesized Ru and C₂N, a two-dimensional organic structure, to verify its performance as a water-splitting catalyst. With the aid of this new catalyst, entitled Ru@C₂N it is now possible to efficiently produce hydrogen.
The technology for producing hydrogen from water requires a good catalyst for commercial competitiveness. These water-splitting catalysts must exhibit high hydrogen conversion efficiency and excellent durability, operate well under low-voltage, and should be economical.
…
…[/font][/font]
eppur_se_muova
(36,260 posts)Roughly 12 tonnes of ruthenium are mined each year with world reserves estimated as 5,000 tonnes.[14]
https://en.wikipedia.org/wiki/Ruthenium#Occurrence
world production in 2010 was 192,000 kg (423,000 lb) {192 tonnes}.[25]
https://en.wikipedia.org/wiki/Platinum#Occurrence
OKIsItJustMe
(19,938 posts)“relatively rare” -vs- “extremely rare” (which is rarer?)
“100 parts per trillion” -vs- “0.005 ppm” (thank you for doing the math)
FWIW: Ruthenium is currently much less expensive (at 40$/“troy ounce” -vs- about $1,000/“troy ounce”):
http://www.platinum.matthey.com/prices/price-tables
NNadir
(33,514 posts)APS, and MRS, respectively the US Department of energy, the National Research Council, the US Geological Survey, the British Geological Survey, the American Physical Society, and the Materials Research Society but, um, but no matter.
J. P. Sykes, J. P. Wright, A. Trench & P. Miller (2016) An assessment of the potential for transformational market growth amongst the critical metals, Applied Earth Science, 125:1, 21-56
In theory ruthenium may be nearly infinitely available however since it is a prominent fission product is used nuclear fuel. (It will be the case in the next 15 years that the supply of another energy critical element, rhodium, will be much larger in existing used nuclear fuel than it is in all the workable ores on earth). This of course, would require the toxic and very dangerous anti-nuke community to come to its senses, a possibility that is extremely unlikely, since they've just spent 50 years of delusional cheering for so called "renewable energy" despite the fact that it hasn't worked, isn't working and won't work, while offering up crazy rhetoric about how nuclear energy is "too expensive" and "too dangerous" blah...blah...blah, even though the United States 30 years ago built more than 100 nuclear reactors, using what is now 40-50 year old technology, while saving hundreds of thousands of lives that would have otherwise been lost to air pollution while simultaneously producing the cheapest electricity on the planet.
(Clearly what has already happened is impossible.)
Could spent nuclear fuel be considered as a non-conventional mine of critical raw materials?
But return to the magical ruthenium supported on C2N nanolayers, even if these kinds of monolayers are extremely challenging to make on anything like an industrial scale.
Very often, people who know almost nothing about science read these kinds of "efficiency" papers and confuse faradaic efficiency with thermodynamic efficiency. It is never true that faradaic efficiency is the same as thermodynamic efficiency, due the requirement of over voltages, which always lose energy has heat.
We don't need no stinking thermodynamics! Energy storage is great!
I mean, after all, they did make a whole gram of this catalyst, meaning it's a good idea to bet the planetary atmosphere on this amazing technological "breakthrough" that will, we swear, make so called "renewable energy" less useless than it's been during the last 50 years of crazed cheering for it.
The process from the original Nature Nanotechnology Letter:
RuCl3 (1.000 g) was first dissolved in N-methyl-2-pyrrolidone (NMP) (50 ml) and placed on an ice bath in a three-necked round bottom flask. Hexaketocyclohexane octahydrate (HKH; 2.811 g, 9.006 mmol) was added followed by the addition of hexaaminobenzene trihydrochloride (HAB; 2.500 g, 9.006 mmol) under nitrogen atmosphere. The reaction flask was allowed to warm to room temperature for 2 h. The ice bath was replaced with an oil bath, which was heated to 175 °C. This temperature was maintained for 8 h. After completion of the reaction, the reaction mixture was cooled to 80 °C and NaBH4 solution (40 ml, 10 wt% in NMP) was slowly added. The mixture was again heated to 175 °C for 3 h. Then the flask was cooled to room temperature and the reaction mixture was precipitated in acetone. The black solid precipitates were collected by suction filtration using a polytetrafluoroethylene (PTFE) (0.5 µm) membrane. The resultant dark solid was further Soxhlet extracted with acetone for 3 days and methanol for 1 day, and freeze dried at −120 °C under reduced pressure (0.05 mmHg). The product was annealed at 800, 900 and 1,000 °C for 2 h under argon atmosphere. For comparison, Co@C2N, Ni@C2N, Pd@C2N and Pt@C2N were also prepared following the same procedure.
Electrochemical characterization
The electrochemical measurements were carried out with a three-electrode cell using a potentiostat (1470E Cell Test System, Solartron Analytical). To prepare the working electrode, 4 mg electrocatalyst and 30 µl Nafion (5 wt% in a mixture of lower aliphatic alcohol and water, Aldrich Chemical) were dispersed in 1 ml water/ethanol (v:v, 3:1) solution by sonication to form a homogeneous ink. The electrocatalyst suspension (5 µl) was loaded onto a rotating disk electrode (RDE) with a diameter of 3 mm (loading amount ∼0.285 mg cm–2). Linear sweep voltammetry was conducted in N2 saturated 0.5 M aqueous H2SO4 and 1.0 M aqueous KOH with a scan rate of 5 mV s−1 and a rotation speed of 1,600 r.p.m. Ag/AgCl (saturated KCl) electrode and graphite rod (Alfa Aesar, 99.9995%) were used as the reference electrode and counter electrode, while RDE with various samples served as the working electrode. The reference electrode was also calibrated (Supplementary Fig. 24). All potentials were referenced to a reversible hydrogen electrode (RHE). Electrochemical impedance spectroscopy (EIS) measurements were conducted to obtain the solution resistance (Rs) with a frequency range 0.1–10,000 Hz at an overpotential of 20 mV (versus RHE). The number of active sites and turnover frequency (TOF) of various electrocatalysts were calculated based on the previously reported method28, 29, 30. All the data presented were corrected with Rs.
I'm sure lots of solar ovens will be around to provide all that heat for all those industrially manufactured catalysts, and I'm sure that the PTFE membranes will not add to the extremely recalcitrant PFOS tragedy now underway all around the planet.
We're saved. Or maybe not. At the Mauna Loa Carbon Dioxide Observatories, despite all these "breakthroughs" the week ending February 12 was um, at 405.91 ppm.
We're doing great! Have a nice President's day tomorrow.