Science
Related: About this forumHidden Perils of Lead in the Lab: Containing...Decontaminating Lead in Perovskite Research.
The paper I'll discuss in this post is this one: Hidden Perils of Lead in the Lab: Guidelines for Containing, Monitoring, and Decontaminating Lead in the Context of Perovskite Research (Michael Salvador,* Christopher E. Motter, and Iain McCulloch, Chem. Mater. 2020, 32, 17, 7141–7149)
When I was a kid, I worked a few years with radioactive iodine-125 to make reagents for an early and once popular form of competitive ligand binding assays; radioimmunoassay kits. The radioactive iodine came in the form of an iodide salt, and the procedure was to oxidize the iodine with a reagent called "Chloramine T," N-chlorotoluene sulfonamide, an oxidant that generates the electrophilic reagent chloroiodine and, inevitably, some free iodine, which can and does volatilize. The chloroiodine would react with aromatic rings - most typically on tyrosine in proteins, and the protein (or other standard) would be thus labeled and detectable at very low levels using a scintillating detector, but some would always escape from the test tubes in which the reactions were performed.
Although iodinations were conducted in the hood it was inevitable, especially because chromatography was generally conducted on benchtops in this lab, that surfaces in the lab would become contaminated with radioiodine, including floors and benchtops, and ultimately the result that scientists working in the lab would themselves become contaminated. Most everyone had a thyroid count after a few months, but one could minimize it by taking iodine supplements, something I encouraged among my peers.
The fun thing was that one could almost always see where and how much one was contaminated with the simple use of Geiger counter, and one could discover quite readily how effectively one's safety practices, gloving, lab coats, lead aprons were working. I had a lot of fun with this. Because I understood radiation better than most people in the lab - these were tools for biological assays after all - I generally took responsibility for handing clean up and waste disposal and I used this experience to learn a lot about contamination and decontamination, which proved useful throughout my career. (If one iodinates proteins in one's skin, one cannot wash it off of course, which is why gloves were always worn, but even these were never 100% effective.)
Of course, working with radioiodine - which is immediately detectable - is good practice for working with nasty metals, like, as is discussed in this paper, lead, which is not immediately detectable and thus is in many ways worse. The same is true of say, Covid-19 viral particles. I would recommend that anyone who is wearing gloves in situations of possible Covid exposure including exposure in the general public and who has not be trained in contamination and decontamination, take a good look at the figure I will post below.
It should be obvious that we have failed miserably to prevent the contamination of the entire planetary atmosphere with carbon dioxide (and for that matter aerosols of the neurotoxins lead and mercury released in coal burning) but that hasn't prevented humanity from doing the same thing over and over again and expecting a different result. Here I am referring to the quixotic effort to displace fossil fuels - although the focus has often drifted into a stupid and frankly dangerous effort to displace infinitely cleaner nuclear energy rather than fossil fuels - with so called "renewable energy," the most popular form being "solar energy."
The failed effort to address climate change with solar energy hasn't lacked for ever more elaborate schemes, and frankly, although I deplore the solar energy industry in general because of its most dangerous feature - it doesn't work to improve the environment - some interesting science has nonetheless resulted from the huge expense, some of which, unlike the solar industry itself, may someday have practical import.
The latest fad in solar research is in the area of perovskites, solids having the following molecular structure:
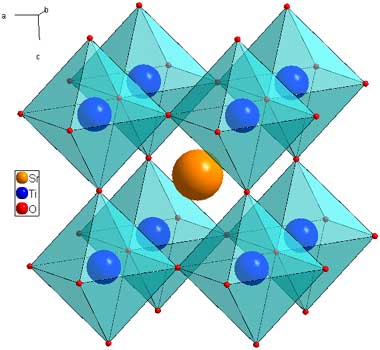
(This cartoon is found on the web pages of the Cava Lab at Princeton University.) One can see that these are octahedra embedded in cubic structures.
The most famous of the perovskites in the endless and continuously failed quest to make solar electricity produce energy on a scale that is meaningful are ternary compounds of cesium, iodine and lead. (Sometimes the cesium can be substituted with small organic ions.) There are actually people who believe that distributing lead for "distributed energy" would be a good idea.
I'm not kidding. The rationale is that "it's not as bad as coal burning" or "it's not as bad as car batteries" which is like saying "breast cancer is not as bad as pancreatic cancer."
Go figure.
Laboratory research is often dangerous and I know from experience that people can get quite cavalier about it. It's a bad idea to get cavalier. People's health can be impacted by the reagents with which they work.
Hence this paper is quite a useful reminder that, um, playing with lead base perovskites can be bad for you.
...Lead(II) salts are precursors of lead-based perovskite semiconductors. In a typical perovskite research lab, spillage of trace amounts of lead powder is unavoidable. Unlike a simple spill of a benign chemical, spills of lead compounds can expose researchers to levels of lead that can be harmful.(3) Lead(II) salts can be toxic and are suspected carcinogens.(4,5) Lead can also accumulate in the body through bioaccumulation in bone and other tissues.(6) It can cause a variety of well documented health problems and can even ultimately result in death.(7) Moreover, lead salts are most commonly provided in the form of fine powders that can easily become airborne and spread across large distances in the lab. Solvents which are commonly used to prepare perovskite formulations, such as dimethylformamide (DMF) and dimethyl sulfoxide (DMSO), can significantly exacerbate the intoxication of lead compounds as they are particularly effective in enhancing skin permeability, thus increasing the risk of absorption via dermal contact.(8) In addition, it is important to realize that contaminated clothing can also transport lead from the lab leading to secondary exposure. Therefore, controlling lead levels at the source is vital in order to reduce lead exposure.
The paper contains a nice table of "permissible" lead exposures:
 ...
...
...

The authors then make this less than reassuring statement:
The authors conduct tests using commercially available colorimetric test swabs and more quantitatively precise and accurate instrumentation, Inductively Coupled Plasma Mass Spectrometery (ICP-MS).
This table gives a flavor for the results:

They give a nice demonstration of how to change and remove one's gloves.
NOTE THAT THIS PROCEDURE IS PROBABLY QUITE USEFUL FOR CHANGING GLOVES POSSIBLY CONTAMINATED WITH COVID, AND FOR THAT MATTER, RADIOACTIVE MATERIALS.

The caption:
They conduct some cleaning experiments:

The caption:
The authors evaluate several commercial lead removal products, but note that the cleaning materials themselves represent a disposal problem.
They also give some scale to the production of 1 GW of lead perovskite solar cells:
By way of full disclosure, the institution where this work was performed was the King Abdullah University in Saudi Arabia. One of the authors, Ian McCulloch, holds a joint appointment at Oxford. I fully expect that some people will therefore assume that the work described herein is tainted. These are the same people who apparently believe that solar panels represent an alternative to the use of petroleum. This is a widely held belief, but it has never been effectively demonstrated anywhere on this planet. It is in fact, a myth, even though well over a trillion dollars has been expended in this century on the solar energy fantasy. In this century the use of oil has proceeded - in terms of energy produced - at three times the rate of wind, solar, geothermal and tidal energy combined, the growing by 34.79 exajoules to 188.45 exajoules, the latter, again combined by 9.76 exajoules to 12.27 exajoules. 12.27 exajoules is just about 2% of world energy demand.
2019 Edition of the World Energy Outlook Table 1.1 Page 38] (I have converted MTOE in the original table to the SI unit exajoules in this text.)
If I were Saudi and wanted to make the oil industry secure, I would encourage the further expenditure of money on solar cells, since they have proved ineffective entirely at displacing dangerous fossil fuels, as demonstrated by the fact, among many similar facts, that recently the arctic has burned owing to climate change, huge tracts of Australia have burned owing to climate change, and huge tracts of the American West Coast are currently burning owing to climate change. The rise in concentrations of the dangerous fossil fuel waste carbon dioxide is accelerating, and as of 2020, has reached an annual rate of 2.4 ppm/year.
Facts matter.
The authors recommend that laboratory work with perovskites be conducted in glove boxes and the precautions be taken to monitor the egress units. They recommend that international safety standards be established for laboratories working on lead perovskite materials.
There are, by the way, methods of safely dealing with hazardous waste from cleaning materials, paper towels, for example, and gloves, about which I've done considerable reading, but to my knowledge, they are not commercially practiced anywhere.
I trust you will have a pleasant Sunday as possible while respecting your own health and safety and those of others.
eppur_se_muova
(36,257 posts)My best training in preventing contamination came from handling thiophenol. The smell is detectable at sub-ppb levels. Everything that touches it has to be immersed in diluted Chlorox before it can leave the hood. Failure to do this will quickly bring down the wrath of one's coworkers (sometimes from quite a distance away). I developed what I term an "airlock mentality" -- the kind of mental habit that keeps you constantly aware of what has touched PhSH, just as someone entering or exiting a spacecraft or submarine is intensely aware of whether or not the airlock is secured. After a while, you can't accidentally remove PhSH-contaminated materials from the hood -- it's just reflexive, automatic.
When I was first learning to handle PhSH I had one day when I had transferred a syringeful into a reaction and successfully cleaned everything afterwards without allowing any detectable traces to escape the hood. Everything seemed fine, and I quit thinking about it for the rest of day. Late at night, after I turned out the light and lay down in bed, I rolled over so that my hands on my pillow were close to my face -- and caught the faintest whiff of thiophenol ! That stuff is really pervasive.
NNadir
(33,512 posts)I worked for some time on the synthesis of sterically hindered thiophenols, selenophenols, and tellurophenols.
For some reason the selenophenols penetrated vinyl gloves. They left a horrible odor on my hands that was more or less impossible to remove even with directly pouring bleach on my hands. I was doing my damnedest to handle them without getting my hands in any kind of contact with them, but obviously I failed to do so, since I ended up with stinky hands.
We were after some inorganic complexes of these nasty things.
The irony was that I had been chasing my future wife for a long time and was finally making progress with her. I was embarrassed as hell about the odor, but it all worked out and she married me anyway.
It was pretty funny, because a lot of men were chasing her at the time, and she ended up with the stinky guy.