Science
Related: About this forumUltralow Thermal Conductivity, Novel Zintl Antimonides, Thermoelectrics, Nuclear Fuel and Fukushima.
The paper I'll discuss in this post is this one: Ultralow Thermal Conductivity and High Thermopower in a New Family of Zintl Antimonides Ca10MSb9 (M = Ga, In, Mn, Zn) with Complex Structures and Heavy Disorder (Alexander Ovchinnikov, Sevan Chanakian, Alexandra Zevalkink, and Svilen Bobev, Chemistry of Materials 2021 33 (9), 3172-3186).
I first found myself thinking about "Zintl salts" some years ago, when considering interactions between the element cesium and lead. These elements do not alloy in a strict sense, rather they form an interesting salt, wherein cesium is oxidized to its common +1 state and lead, a metal, is reduced to a remarkable anion having a cluster structure:
Deltahedral Clusters in Neat Solids: Synthesis and Structure of the Zintl Phase Cs4Pb9 with Discrete Pb94- Clusters (Evgeny Todorov and Slavi C. Sevov Inorganic Chemistry 1998 37 (15), 3889-3891)
A figure from the above:
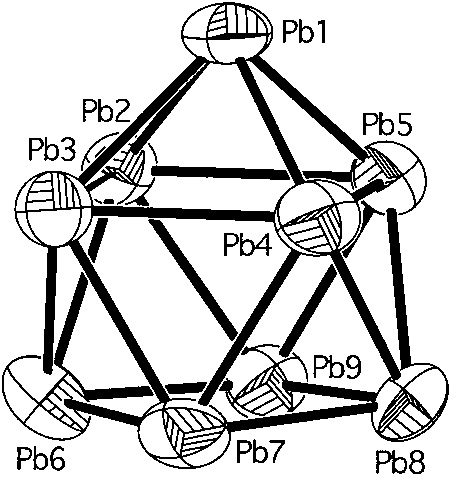
The caption:
Thermoelectric devices generate electricity directly from heat; no moving parts are involved. They are extremely reliable devices, as evidenced by, among other devices, the Voyager Space Craft which is powered by a thermoelectric "RTG" which has been sending signals from deep space for 44 years, nearly half a century. The power source on the Voyager space craft derives its heat from the decay heat from plutonium-238. Similarly powered devices have been utilized for the many space missions, most recently on the Mars Rover Perseverance. To continue utilization of these types of devices, the United States has resumed the production of plutonium-238, supplies of which ran out.
The root cause of the failure of the nuclear reactors at Fukushima to survive a tsunami better than the surrounding cities - which also did not survive the tsunami although their failure and the connected deaths excited little interest compared to the interest in the failure of the reactors to survive - was connected to the fact that diesel generators won't operate under water. In the case where the reactor is not producing electricity - armatures and steam turbines won't turn under water, back up power is necessary, and, on almost every nuclear reactor in the world, this pack up power is fueled by diesel generators. The coolant pumps for the reactors, which do operate under water thus had no source of back up power when the reactors shut down, with the result that the decay heat in the fuel caused temperatures to rise to a point at which the following reaction takes place: Zr + 2H2O ZrO2 + 2H2. (The fuel rods are made of an alloy of Zirconium called "Zircalloy-4." ) The generated hydrogen mixed with air and was ignited by the heat, causing an explosion.
Like most thermal devices designed to produce electricity, a thermoelectric device is designed to operate over a temperature gradient and the thermodynamic efficiency of the device is related to the establishment of the difference in temperature between the hot and the cool region. The greater the difference in temperature between the hot and cool regions, the greater the thermal efficiency. This is why when one looks at a picture of an RTG on a space craft, the device always has cooling fins; these are designed, even in deep space, to radiate heat.
It follows that an RTG that is placed under water, because of water's high thermal conductivity as well as its high heat capacity can be expected to function at higher efficiency than when cooled by air. This effect is even more pronounced with flowing water.
Ironically, one concern after the reactors were destroyed was the state of the used fuel in "cooling ponds" because of the lack of recycling cooling water. The fuel remained intact (as it was designed to do) in borated water but it was a "concern" and certainly journalists, whose income is proportional to the amount of hysteria they can generate, for example "her emails," prominently hyped this "concern." The reason is that used nuclear fuel even after it is removed from the reactor still is generating significant heat in exactly the same way that an RTG in space is doing, from nuclear decay (as opposed to critical nuclear fission.)
When devices fail, the mode of failure is instructive on how to make them less prone to repeat failure. One approach to preventing another event like Fukushima would be to do away with diesel engines for power back up and replace them with RTGs powered by decay heat which is readily available at nuclear power plants. It's called, using the idiomatic cliche, "killing two bird with one stone." (The switching electronic devices also failed at Fukushima, so making these immune to submersion would also be a good idea.)
The heat load associated with used nuclear fuel - it changes rapidly with time - is covered in a marvelous Masters thesis (under her birth name) by Dr. Kristina Diane Yancey Spencer, now at INL, when she was a graduate student in the nuclear engineering department at Texas A&M, Nationwide Used Fuel Inventory Analysis.
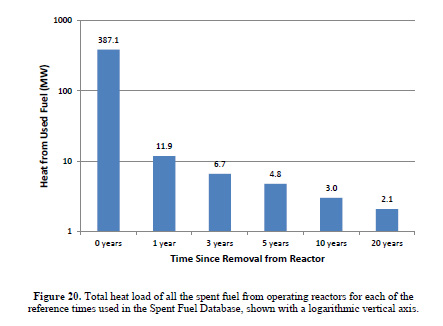
The caption:
(The power levels here refer to the total amount of fuel removed in a given year from all US reactors, and thus this heat is not located in a single place.)
The next figure is more interesting, and I will discuss it below:
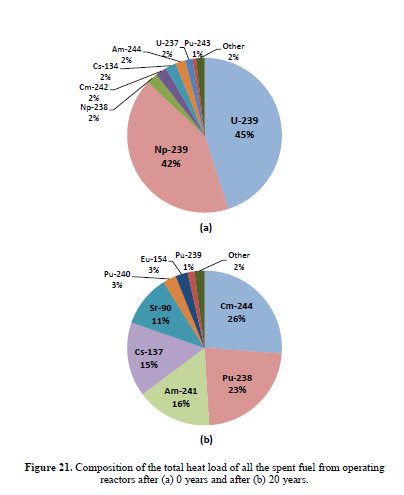
The caption:
(She also published the same figures in a paper with her advisor:
Quantification of U.S. spent fuel inventories in nuclear waste management: Quantification of U.S. spent fuel inventories in nuclear waste management (Kristina Yancey, Pavel V. Tsvetkov, / Annals of Nuclear Energy 72 (2014) 277–285)
I don't use the term, "nuclear waste," but prefer the term "nuclear resources," since the plutonium in this so called "waste" is critical, in my mind, to saving the world, and sometimes, perhaps more often, I also use the term "used nuclear fuel," but OK, let me not quibble with Dr. Yancey Spenser, a scientist I admire. The point is that language matters, even when stating facts that matter.)
The heat load at time T=0 is not really recoverable after a reactor shuts down, since the largest portion of it comes from the decay of U-239, which has a half life of 23.45 minutes. The maximum activity associated with U-239 and it's decay daughter, Np-239, which has a half life of 56.544 hours, is reached after 169.5 minutes (2.82 hours), whereupon the ratio between Np-239 and U-239 is 144.7. This decay heat of course, is part of the heat generated during a reactor's operation, but it seems unlikely that it would be possible to capture it in a thermoelectric device after shutdown, and in any case, the heat output would rapidly decline. Even though I personally have spent a lot of time contemplating fast separations of fresh used nuclear fuel while it's still very hot, even I can't imagine recovering this energy. The heat load a couple of weeks out is far more interesting, but let's leave that discussion for another time.
What I'd like to focus on is figure 21b, the heat load 20 years out, particularly the five isotopes providing the majority of the heat load, Cm-244, Pu-238, Am-241, Cs-137 and Sr-90. Two of these have been incorporated into thermoelectric devices, Pu-238 for space missions, and historically, for pacemaker batteries implanted in chests, and Sr-90, which powered some arctic lighthouses in the former Soviet Union. In a continuous closed fuel cycle for power production (and/or high temperature thermal processing) the Pu-238 would be carried along with the Pu-238 and end up in the next generation fuel, while making it less usable, if usable at all, for weapons production.
In fast reactors - the kind I favor - Am-241, which has sometimes been suggested for use in thermoelectric space devices, can operate as an excellent nuclear fuel. It has a significantly lower density than plutonium, with the effect of increasing heat exchange area and a higher critical mass. (I discussed the critical mass of americium isotopes here: Critical Masses of the Three Accessible Americium Isotopes.) During operation, it generates two plutonium isotopes of great value for non-proliferation of nuclear weapons, Pu-238 (via Cm-242) and Pu-242. Although, again, it can be used in thermoelectric devices, and has been evaluated for use as such by the ESA for space missions designed to last even longer than the Voyager missions, the heat load is significantly lower than for Pu-238, 0.115W/g vs. 0.568W/g for Pu-238.
I have convinced myself - with considerable enthusiasm - that the chemical and radiation value of cesium-137 is way to high for solving otherwise intractable environmental problems, in particular the atmospheric rare but potent climate change gases, as well, albeit to a limited extent, extraction of carbon dioxide from the air. This is a conversation for another time, but it would be a waste of perfectly good Cs-137 to simply limit it to thermoelectric devices.
Although Strontium-90 has historically be utilized in thermoelectric devices, it also suggests uses other than that role because of some remarkable nuclear, metallurgic, and chemical properties.
This leaves Cm-244 as a potential thermoelectric fuel to displace diesel back up generators at nuclear power plants. The half-life of Cm-244 is 18.11 years, during which it decays to the useful non-proliferation plutonium isotope Pu-240. It's thermal output, which can be compared to Pu-238 and Am-241 as described above, is higher, 2.83W/g.
From Dr. Yancey Spenser's Master’s thesis above, to estimate how much Cm-244 is available from the heat load described and its thermal output, and the use of the simple radioactive decay law. It is important to note at this point that Dr. Yancey Spencer's estimates produced during her graduate school years are just that, estimates, derived from in silico calculations using the ORIGEN nuclear codes, although she provides an excellent analysis of the common commercial nuclear fuel assemblies, reactor types, and enrichments. Nevertheless, these "seat of the pants" calculations definitely have use. Using her data, I calculate that in a given year, freshly removed used nuclear fuel contains about 1.6 tons of Cm-244, albeit in extremely dilute forms, and, again, using back calculations, that an inventory of used nuclear fuel going back twenty years, will be about 23.5 tons, accounting for annual decays. The heat output of this inventory 66.4 Megawatts thermal.
However the thermodynamic efficiency of thermoelectric devices can be rather low, even lower than the poor energy efficiency of solar cells, where people get excited about energy efficiencies greater than 20%.
There's a very nice presentation from a post-doc symposium held at Cal Tech that gives an overview of thermoelectric materials, sponsored by NASA's Jet Propulsion Laboratory:
Overview of Radioisotope Thermoelectric Generators: Theory, Materials and New Technology.
For convenience, I'll post a few graphics from that presentation here. The first is the equation for a parameter known as "ZT," which is actually a single variable, and is not a product of two variables:
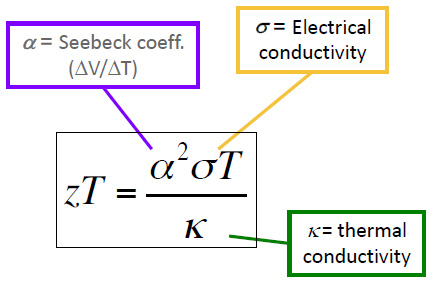
It is known as the "figure of merit" for thermoelectric devices. The "figure of merit" for a thermoelectric device, relates to its thermal efficiency according to the following equation, which includes both Carnot terms (as in a mechanical heat engine) related to the heat source and the heat sink, that is ambient temperatures, as well as a term related to the value of ZT:
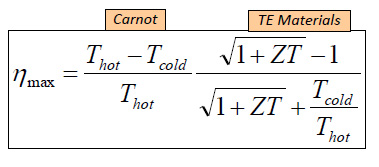
Enhancing the value of ZT is the goal of much research. Returning to the equation for it, we see that its value climbs as the thermal conductivity decreases, which is the point of the value of the ultralow thermal conductivity discussed in the paper referenced at the outset of this post, the reason that "zintl antimonides" are worthy of consideration.
The NASA people provide some insight to where we are with advances in terms of what is commercially available, and what has been developed on a lab scale...
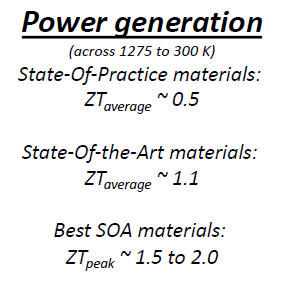
...and then produce this nice graphic showing how thermal efficiency would vary for different ZT values over temperature ranges:
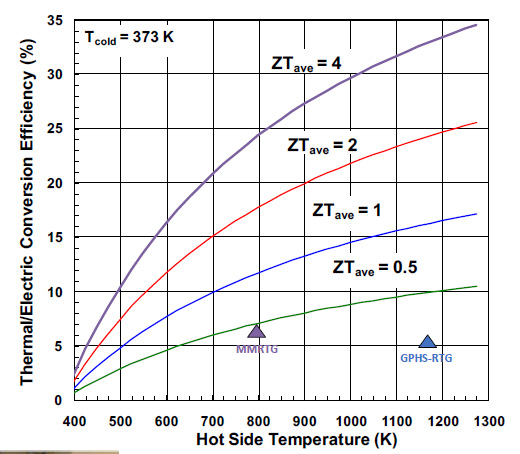
Note that the "cold" temperature, the heat sink, is given in this graphic at 373K on the Kelvin (thermodynamic) temperature scale. This translates to 100 degrees Celsius, the boiling point of water. I'm not sure why this value was chosen, but can speculate that the decay of plutonium-238 on spacecraft is not only required to generate electricity, but also to heat the components of the instruments via conductive materials, thus requiring a higher "cold" temperature. The "MMRTG" is the device that is currently powering the Opportunity rover now investigating the planet Mars. Note that the MMRT has rather low thermal efficiency, around 7%. There may be many reasons that NASA chose the thermoelectric material for the MMRT, considerations of weight, longevity, resistance to thermal stresses, brittleness, etc., conditions which may not and probably wouldn't apply on Earth bound devices.
The types of materials that maximize ZT are semiconductors, and the "hole carriers" in the following graphic refer to the "n type" and "p type" "holes" in the semiconductor field, as shown in the following graphic:
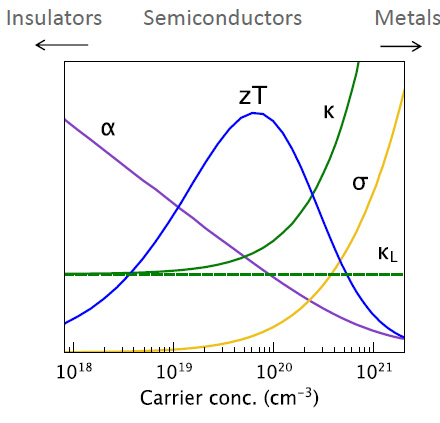
It is worth noting that although the solar industry has proved, is proving, and will always prove useless in addressing climate change, the research conducted to develop solar cells is not useless. There are some rather exotic hole carriers that have been developed for that industry, for example, the interesting organic molecule spiro-OMe-TAD. The synthetic precursors of this molecule are petroleum based, but in a nuclear powered world, where chemistry would be dominated by the use of syngas, anything now obtained from petroleum will be accessible from the reduction of carbon dioxide.
I'd like at this point to return to what happened at Fukushima and Dr. Yancey Spenser's graphics above. The fuel rods in the cooling ponds that were of most concern in the hysterical days after the natural disaster which destroyed buildings, homes, cars and human lives - not that we, as a whole, give a shit about the roughly 20,000 people who drowned or were crushed by collapsing buildings, but would rather prattle on that someone might die from radiation exposure someday - were those which were the hottest, specifically those which had most recently been removed from the reactors. As shown in the bar graph, the first reproduced here from her thesis, the one with the logarithmic y-axis, the heat load falls rather rapidly in the first year of cooling, by more than an order of magnitude.
I'd like to turn to two heat producing nuclides near and dear to my heart shown in her first pie chart, the one for year zero, Cm-242 and Cs-134.
The latter is not a major fission product because of the naturally occurring isotope of xenon, Xe-134, which occurs earlier in the beta decay chain occurring among the fission products, all of which are neutron rich. Xe-134, according to the nuclear stability rules, should be radioactive, since the lower mass stable isotope Ba-134 exists, but if it is radioactive, the half-life is so long that its radioactive decay has never been detected. In a nuclear reactor, the Cs-134 isotope which delivers considerable heat in freshly removed used nuclear fuel is formed by neutron capture in the only stable non-radioactive isotope of cesium, Cs-133. (I have convinced myself that in certain kinds of fluid phased reactors, the isolation of nearly pure non-radioactive Cs-133 is possible)
According to Dr. Yancey Spencer's ORIGEN based in silico calculations, hundreds of kilos of Cs-134 are generated in a year, and the sum of power production at specific T=0 time points is on the order of 8 MW. Half a year later, the power output is just under 7 MW.
Cm-242, has a similar power output at T = 0, around 8 MW although much less of it is available, around 10 kg, but after half a year the power has fallen to under 4 MW.
In addition there are many other fission products with relatively short half-lives, on the order of months, that put out significant heat, Ru-106, Ce-144, etc, which rapidly decay into valuable non-radioactive materials.
Using the formula provided above for thermal efficiency, and a "state of the art" thermoelectric material with a ZT of 1.1, a temperature for the heat output of decaying nuclides of 800K and ambient temperatures of 273K, the thermal efficiency is on the order of 37%. (Note that I’m being a little disingenuous here since ZT can vary with temperature, since thermal conductivity and electrical conductivity are generally functions of temperature.)
The temperature at which zirconium reacts with steam to generate hydrogen, which is what took place in the Fukushima reactors is on the order of 1500 °C or higher, and if follows that in the cores of the Fukushima reactors these temperatures were observed when the heat sink failed, despite the placement of the control rods to shut the chain reaction. These temperatures almost certainly resulted, as we've seen, from the decay of of short half lived nuclei like U-239 and more importantly, Np-239 and Np-238, as well as fission products with half-lives from a few weeks or months to a few years.
With all this in mind, and given the concern that the fuel rods in the cooling ponds might similarly fail - they didn't - it is reasonable to assume that the cooling fuel may have had a considerable thermal output, quite possibly significantly more than 10 MW.
A thought experiment: Suppose instead of putting the used nuclear fuel in a cooling pond, where its energy was wasted, the fuel rods were placed in a molten salt bath surrounded by a thermoelectric material, say one with a ZT value, as above of 1.1, with the decay heat temperature at 800K. Fresh used nuclear fuel with a thermal output of 10MW would thus be providing about 3 MW electrical energy. I am not familiar with the detailed design of the Fukushima BWR other than to know that the diesel generators were located in the basement of the reactor buildings, and thus were swamped. However for other reactors, these generators seem to be on the order of 1500 kW to 3000 kW, 1.5 MW to 3 MW. The power supply would be continuous, and uninterrupted, since the device would have no moving parts.
Indeed, in a molten salt bath of certain types - there are huge number of possibilities beyond the famous, and to my mind of dubious desirability FLIBE - anodic dissolution of the fuel rods to obtain their contents. Some of the radioactive materials would be more useful in settings other than merely providing heat. Under these conditions, either direct separations or separations using aliquots would allow for the separation of particular radioactive elements for other uses.
For instance two of the radioactive isotopes of cesium, both 134, discussed above, and the better known 137 isotope, are associated with powerful gamma rays.
Combustion power plants using either dangerous fossil fuels, biomass or trash as an energy source take in air and discharge it powerfully dirtied beyond the climate gases already there from earlier combustion events. I have convinced myself, and hopefully my son, that it is possible to build a power plant, nuclear fueled, which takes in dirty air and discharges it cleaned, with the powerful warming gases CO2, methane, N2O, hydrofluorocarbons and residual CFCs, and indeed sulfur hexafluoride, all removed as well as aerosol particulates, aerosol microplastics, sulfates and sulfites. Having powerful gamma radiation, such as that associated with Cs-137 and Cs-134 might well contribute to such a system, but that's for discussion elsewhere, except to say that there is impetus for doing such separations. The high energy to mass density of nuclear fuels, coupled with Bateman equilibria that places limits on the amount that can form before radioisotopes decay as fast as they form, will mean that there will never be vast amounts of these isotopes, but nevertheless, in continuous flow processes, this gamma radiation might make a significant impact.
The bulk of used nuclear fuel is unreacted uranium. Once through or multiply recycled uranium is somewhat more valuable, at least to my mind, than natural uranium, as it contains the U-236 isotope that does not occur in ores. This isotope, which can be thought of as an inert uranium isotope, since it is not appreciably fissionable except in the epithermal region, and even there, marginally so, and thus has intrinsic nonproliferation value. Reuse of recycled uranium leads to the accumulation of valuable isotope neptunium-237 Removal of this uranium for use in breeder reactors will result in concentration of heat producing isotopes and elements like curium (which is a minor impurity in used nuclear fuels, albeit responsible for much if the generated heat). The point is this: Over many decades of operations at a nuclear power plant considerable amounts of heat generating isotopes not useful in other settings, might thus accumulate, and establish an array of electrical power producing devices that lack moving parts.
When aircraft fail, or for that matter, when automotive systems prove unsafe, neither aircraft nor automobiles are abandoned as technologies. The flaws are engineered away, or procedures put in use to prevent recurrence. The nuclear industry, while not risk free any more than any other industry is risk free, is spectacularly safe when compared to the very dangerous fossil fuel industry, which kills people in vast numbers when it operates normally, never mind in failure modes.
It is possible, I suggest, to engineer away events like Fukushima. It is not, however, possible to engineer the destruction of cities by seawater, but we can minimize that risk to some extent by addressing climate change.
And let's be clear on something. We are decades into cheering by anti-nukes and others into the theoretical value of so called "renewable energy." It did not work. It is not working. It will not work. And by "working" I mean, working to address climate change. The theory was not borne out by experiment, and, as this is the Science forum, if a theory does not agree with experiment, it is the experiment that is discarded; it is the theory.
The experimental results of the expensive "renewable energy will save us" theory are in: The use of dangerous fossil fuels is rising, not falling. The reliance on dangerous fossil fuels is not merely killing people continuously. It’s killing the entire planet.
Thus engineering away the comparatively minor risks of events like Fukushima, there is a strong impetus for the development of thermoelectric devices, and thus it is important to study materials with extremely low thermal conductivity, indeed for other reasons, for example, the construction of devices capable of utilization in high temperature exposure to increase thermodynamic efficiency, this to reduce the cost of energy so as to extend its benefits to those who lack them by reducing costs and increasing access.
From the introductory text of the paper cited at the outset of this post:
A special class of materials that has been actively explored as promising candidates for thermoelectric applications are Zintl phases—valence-precise intermetallic compounds with polar chemical bonding.(3−6) The charge balance in these materials is achieved by electron redistribution between different parts of the crystal structure so that the constituting atoms adopt a stable closed-shell electron configuration. Typically, such compounds demonstrate semiconducting properties with narrow electronic bandgaps and tunable electrical resistivity. At the same time, high structural complexity enables low phonon group velocities and efficient phonon scattering and hence low thermal conductivity.(3−11)
Most Zintl phases of relevance for thermoelectric research belong to the chemical families of tetrelides and pnictides, i.e., compounds with the elements of groups 14 and 15, respectively. One of the highest-performing materials within the pnictide family is Yb14MnSb11, with the maximum zT value approaching 1.2 at T ≥ 1000 K...
Yb, ytterbium, is not what we call an "earth abundant element," it is a minor constituent of lanthanide ores. It is not a fission product. Antimony, Sb, is also not an "earth abundant element" but it is a fission product. In the fast fission of plutonium, the kind of approach to plutonium utilization I favor, about 0.25% of fissions produce a mass number associated with antimony. Antimony in fresh used nuclear fuel will contain the radioactive isotope Sb-125, but the half life of this isotope is relatively short, 2.76 years, whereupon it decays into a stable isotope of the similarly valuable element, tellurium, which is also an element of concern and which is also valuable in some thermoelectric settings. Thus aged used nuclear fuel, accumulated in the 1970s and 1980s is a potential source of both of these elements without requiring mining.
The paper's introduction continues later:
Neither gallium (Ga) nor Indium (In) are "earth abundant elements. Indium is a fission product, but only one of the two natural isotopes is present, the radioactive one, the 115 isotope, owing to the extremely long half-life of the cadmium 113 radioactive isotope. Indium is one of the rare elements whose radioactive component is dominates its natural ores. The indium in your touch screens, including that in your cell phone, is radioactive, but the half-life is so long that it doesn't matter. Thus it is possible to isolate indium from used nuclear fuel, although its concentration is very low, about 0.1%, although such concentrations are found in ores that are used to provide this element for touch screens and for unsustainable solar cells of the CIGS type.
However, Mn and Zn, are relatively abundant, although there is concern about minable zinc ores. Zinc may ultimately be required to be obtained from very dilute streams, requiring significant energy inputs.
The poor (and desirable) low thermal conductivity is a function of relative disorder, isolating the phonons through "phonon scattering" - phonons are the structures in matter that transmit heat and sound vibrations.
Some pictures from the text:
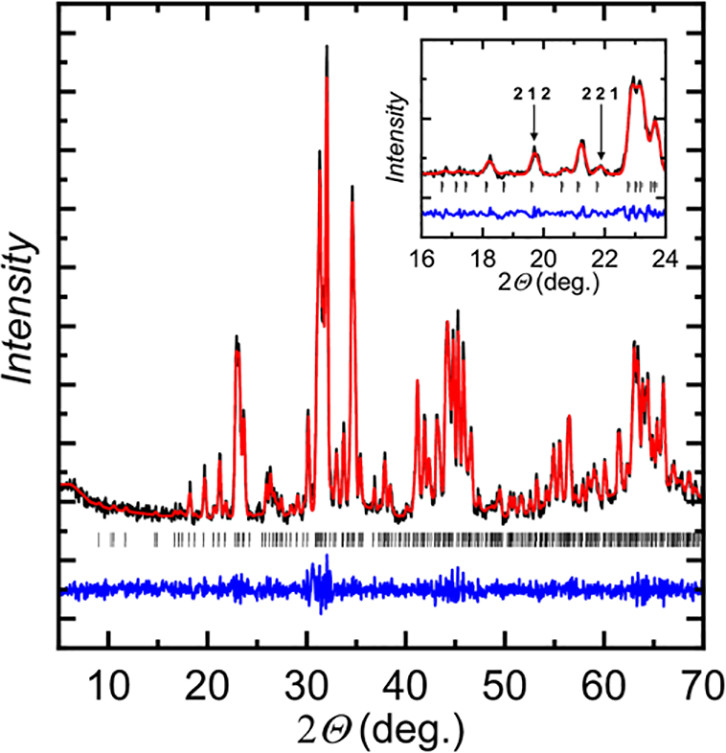
The caption:
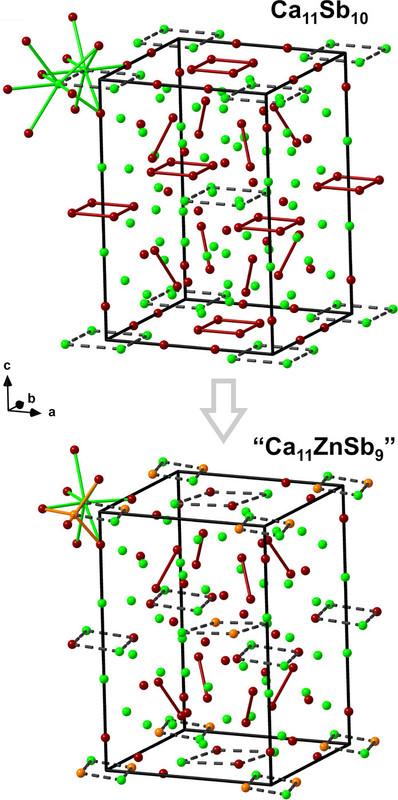
The caption:
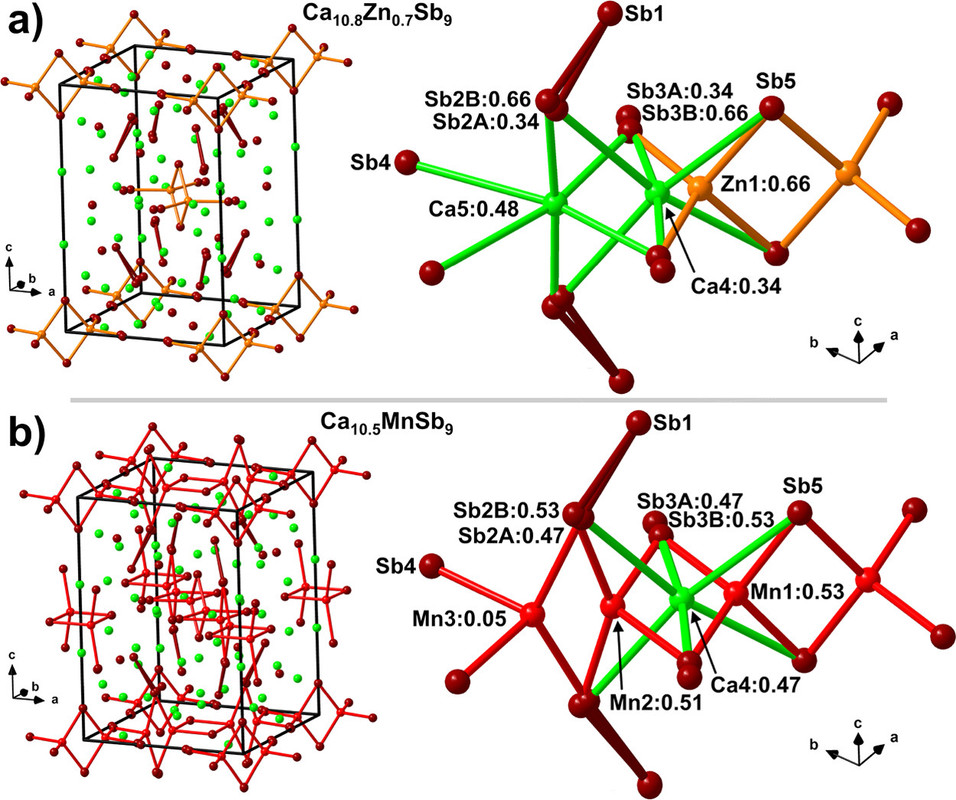
The caption:

The caption:
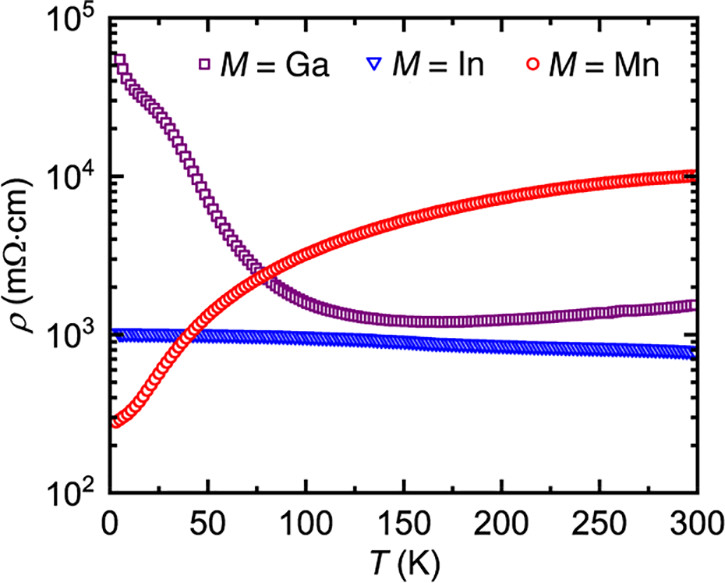
The caption:
In this case the earth abundant elements, Mn and Zn perform better than the less available Ga and In, but this does not necessarily carry through to thermoelectric performance.
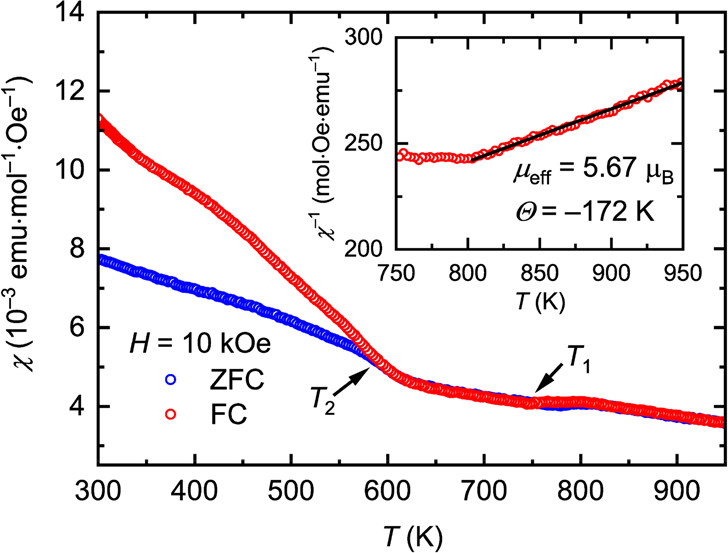
The caption:
The actual performance of these materials as thermoelectric materials is nothing about which to write home, but this said, the purpose of this investigation is not to develop a definitive material but rather to suggest a path to optimization. The development of nanostructured materials, including perhaps "hole carriers" suggest a value.
As for the performance of the investigated materials as thermoelectric materials, again, nothing about which to write home, there's this graphic:
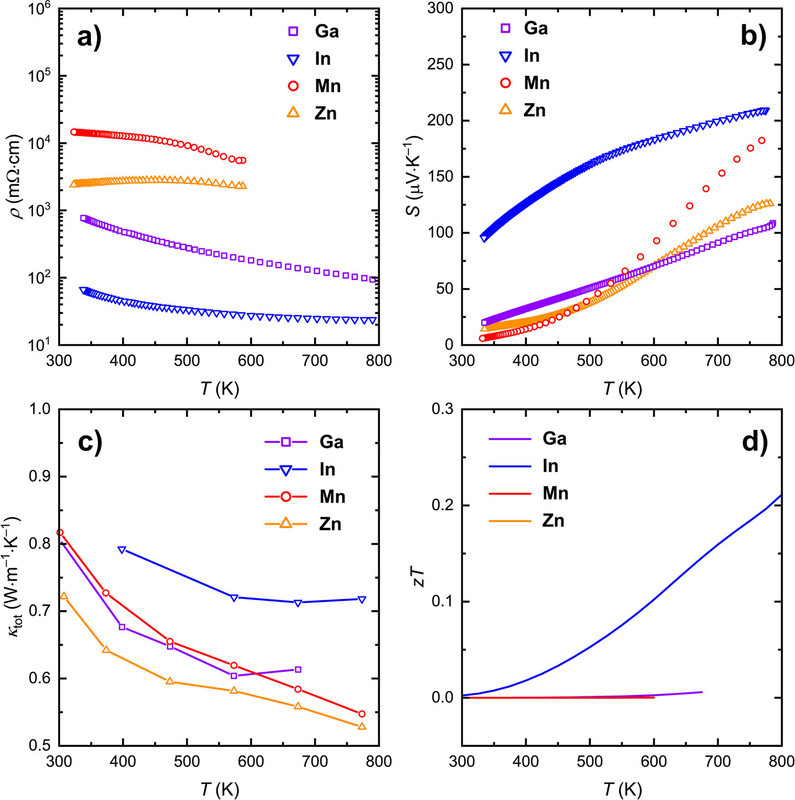
The caption:
This is indeed, worthy research. Almost every issue of the journal Chemistry of Materials includes a few papers discussing thermoelectric devices, although I have been struggling to keep up with this publication in recent weeks.
This same issue, issue 9 of this year, includes another paper discussing the theory of thermoelectric devices, interestingly including those dependent largely, if not entirely, on earth abundant elements:
Key Role of d0 and d10 Cations for the Design of Semiconducting Colusites: Large Thermoelectric ZT in Cu26Ti2Sb6S32 Compounds (Takashi Hagiwara, Koichiro Suekuni, Pierric Lemoine, Andrew R. Supka, Raju Chetty, Emmanuel Guilmeau, Bernard Raveau, Marco Fornari, Michihiro Ohta, Rabih Al Rahal Al Orabi, Hikaru Saito, Katsuaki Hashikuni, and Michitaka Ohtaki Chemistry of Materials 2021 33 (9), 3449-3456)
This paper describes materials with a ZT as high as 0.9, albeit titanium and copper materials doped with germanium, which cannot be considered an earth abundant element.
Again, there's a lot of research in this area. My personal interest in selecting the paper I discussed out many possible such papers was more connected with my interest in thermal insulation materials for high temperature applications, but I fully appreciate the need to understand these materials in thermoelectric settings, which I discussed at greater length.
This post is, admittedly, esoteric, particularly for a political website, but I write these things to teach, if not anyone else, myself.
I trust you are having a pleasant weekend.