NNadir
NNadir's JournalThe astounding cadmium intake associated with rice in Southern China.
Recently in this space, I reported on the development of an inexpensive test for cadmium contamination developed by Chinese scientists, noting that China has a very serious problem with the contamination of their rice crops with this dangerous heavy metal.
That post is here: Fast, accurate determination of cadmium contamination without expensive instrumentation.
I have been generally aware of the cadmium contamination of Chinese rice over the years because, in something of a gadfly role, I question the rote assumption that so called "renewable energy" is actually safe and sustainable - it is neither, nor is it in fact even "renewable" - and I believe that simply by saying the word "renewable" in connection with energy people tend to abandon critical thinking and in fact, stop giving a rat's ass about the consequences of wishful thinking, the kind of wishful thinking that kills people.
Uncritical belief in the cleanliness and sustainability of so called "renewable energy" is our creationism on the left.
(The planet in the last ten years squandered over a trillion dollars on solar energy - a fig leaf for the gas industry without which solar energy would be even more useless than it is - with the result that the degradation of the planetary atmosphere accelerated to unprecedented levels. The solar industry has not worked, is not working and will not work to prevent climate change or to save the lives of even a minute fraction of the seven million people who die each year from air pollution.)
Whatever.
Having stumbled on that paper in my regular reading, I decided to update my understanding of the cadmium situation in China by picking up some references in the paper I discussed in the original post, and I came across a paper today that was truly astounding.
The new paper is here: Assessment of dietary cadmium exposure: A cross-sectional study in rural areas of south China (Yang et al, Food Control Volume 62, April 2016, Pages 284-290)
The abstract and "highlights" available in the link are obvious even if one cannot access the paper directly: The Chinese in the most contaminated areas are accumulating 340 mg of cadmium over their lifetime. For reference this is 38% of the LD50 dose, an LD 50 dose being the dose at which half of the test animals experiencing this level will die immediately. The chronic effects of cadmium exposure include not only death, but debilitating organ failure.
In the text of the paper the authors note that some of the levels of cadmium exposure exceed contamination levels in those regions of Japan where cadmium exposure lead to the outbreak of "Itai-Itai" ("Pain-Pain"![]() disease characterized by severe osteoporosis and renal failure and increase mortality. The "Itai-Itai" victims experienced contamination levels averaging 0.59 ug/g in their rice; the Chinese in the surveyed regions in this paper are eating rice with 0.64 ug/g.
disease characterized by severe osteoporosis and renal failure and increase mortality. The "Itai-Itai" victims experienced contamination levels averaging 0.59 ug/g in their rice; the Chinese in the surveyed regions in this paper are eating rice with 0.64 ug/g.
The analytical method employed for measurement was state of the art, an Agilent 7700 ICP/MS utilizing microwave nitric acid digestion.
An estimate of the number of people likely to experience severe health consequences has been made in another paper, this one:
Applying Cadmium Relative Bioavailability to Assess Dietary Intake from Rice to Predict Cadmium Urinary Excretion in Nonsmokers (Li et al, Environ. Sci. Technol., 2017, 51 (12), pp 6756–6764)
I quote:
"Urinary Cd has been associated with increased diabetes, hypertension, and even cardiovascular disease mortality.4,5 The incidence of osteoporosis at 2.4% associated with chronic Cd exposure from a smelting-impacted area in southeast China has-been correlated with urinary Cd < 2.0 μg g−1 creatinine.41 Considering the high predicted urinary Cd concentration of 4.77 μg g−1 creatinine in Hunan province with 67 million population, ∼1.6 million population may be at risk of osteoporosis due to rice consumption based on the low incidence of 2.4%. These findings suggest that ingestion of rice can be a health risk for populations in China, especially in southern China. Increased attention should be paid on food safety where both Cd concentration and Cd−RBA are important. Furthermore, strategy should be developed to modulate dietary Cd exposure via decreasing Cd−RBA in rice."
RBA = Relative bioavailability.
Over the years, I've had the misfortune to listen to people claim - in complete ignorance - that so called "renewable energy" is safer than nuclear energy. This is nonsensical and is based entirely on selective attention. I recently had to listen the unpleasant and frankly insipid announcement that the collapse of a tunnel at the Hanford nuclear reservation - a weapons plant - "proved" that nuclear power was unsafe. The tunnel collapse killed or injured no one and yet it was international news.
One hears a lot about Fukushima radiation, even though the death toll thus far is zero from radiation; in the same event thousands of people were killed by, um, seawater. Yet rising seas are ignored, radiation scare mongering is an international pass time.
One never hears, by contrast, about the trichloromethylsilane explosion at a Mitsubishi plant, also in Japan, trichloromethylsilane being an intermediate for some types of solar cells. The explosion killed 5 people immediately, and severely injured 15 people. The trichlorosilane explosion was not international news. Nobody gave a rat's ass about it, just like no one gives a rat's ass about the thousands of fatal natural gas explosions over the years; natural gas being the fuel without which the entire so called "renewable energy" scam would collapse in a New York minute.
Risk inasmuch as it involves threat to human life is described by WHO as being measured in DALY, "Disability Adjusted Lost Years." In this sort of calculation, a person killed instantly counts more than a person who might develop cancer from exposure in thirty years.
But don't kid yourself; the thirty year exposure limit is not zero for the solar scam. Cadmium is a carcinogen as well as a systematic poison. It has no half life. It never goes away.
The solar industry is trivial; the nuclear industry is not. One wonders, should the solar industry soak up so many more tens of trillions of dollars useless to even approach 1/5 of what nuclear energy has produced year after year after year for nearly half a century using technology developed in the 1960's, what the "solar" death toll might be.
It will not be small:
The cadmium situation in China suggests that the death toll for this still trivial form of energy is already higher than all the deaths associated with Fukushima, Chernobyl, yada, yada, so on and so on. These deaths will not be erased because currently no one - other than scientists in China - gives a shit about them.
The unacceptable death toll from the solar industry - should anyone ever pay attention to how "safe" this "green" scam is - is nonetheless still small when compared with the 70 million people who die each decade from air pollution while some us, wait like Estragon waiting for Godot, for the so called "renewable energy" industry to actually do something. It hasn't, it isn't, it won't.
The worst risk of "renewable energy" is that it is ineffective, and thus allows the killing to go on.
Nevertheless, to the extent we "distribute" cadmium telluride and cadmium selenide solar cells in this country, we will have the same hell to pay as the Chinese are paying now as they mine this stuff so we can be "green." The payment will come due during the lives of our children and grandchildren and their children and grandchildren, the people we screwed by doing nothing about the environment at all other than make glib predictions about how wonderful their energy sources will be since we expect them to do what we no idea how to do ourselves, live in some fantasy "renewable" nirvana.
Enjoy the upcoming holiday weekend.
Energy efficient electrolytic recovery of chlorine from waste HCl.
Here's a nice paper I came across this afternoon on addressing a very serious industrial problem, what to do with waste HCl, hydrochloric acid: Sustainable Non-Noble Metal Bifunctional Catalyst for Oxygen-Depolarized Cathode and Cl2 Evolution in HCl Electrolysis (Tharamani C. Nagaiah et al, Chem. Mater., 2017, 29 (10), pp 4253–4264).
The authors state the problem very well in the introduction:
The increased production of excess HCl as a byproduct cannot be utilized effectively only by employing it in the production of PVC or for other small manufacturing industries. Moreover, many small-scale industries in India and other developing countries simply quench the produced HCl with lime. The option of neutralizing the excess HCl is inadmissible for obvious reasons. Therefore, an intelligent way of valorizing the HCl produced as a byproduct is the recycling strategy for its conversion into high-purity Cl2 in order to make the associated processes sustainable. Chlorine production at present is primarily based on HCl electrolysis, which is now a substantially imperative methodology that involves the generation of hydrogen at the cathode (E0 = 0.00 V) and chlorine at anode (E0 = 1.36 V) with an overall reaction potential of −1.36 V.(2, 3)"
The authors don't note this in their paper, but a very common approach to chlorine production over the years has been to utilize mercury electrodes, which has lead to a very serious mercury contamination problem - commercial laundry bleach can often contain mercury - which along with medical waste and the worst environmental mercury release scheme of all, the use of dangerous fossil fuels as a source of energy, has contributed to serious mercury contamination of human beings.
(I sometimes speculate whether "mad hatter disease" is partially responsible for the willingness of a major North American country allowing itself to be "lead" by an inane and possibly insane corrupt orange monster of extremely low intelligence.)
In any case, they point out that one approach to regenerating chlorine is to oxidize it with oxygen with what is known as "an oxygen-consuming cathode known as oxygen depolarized cathode (ODC)" Here the oxygen gas is reduced to water with the addition of 4 protons and the release of 4 electrons, and at the anode, 4 chloride ions are oxidized to chlorine gas.
Unfortunately most of the ODC's contain expensive noble metals like platinum or rhodium. The authors exploit some very modern materials science to construct a new kind of ODC based on oxidized carbon nanotubes, zinc and tungsten.
Here's a picture of the structure of the catalyst:

The structure is a layered arrangement of polyvinyl imidazolium cationic polymers, oxidized carbon nanotubes and a zinc polyoxo tungstenate.
They claim that besides avoiding expensive metal catalysts (often not available in the third world as they describe) the approach saves about 30% of the energy required to regenerate chlorine from HCl using this system.
The disposal of HCl is a big problem in the first world as well. In many places HCl has been "deep welled" - that is dumped into deep bore holes.
This is hardly acceptable and if scalable, this is a very cool solution to the problem.
This is an obscure, but nonetheless important issue.
Have a nice Sunday evening.
Neil Bartlett's superpowerful oxidants NiF6- and AgF4- and the preparation of RhF6.
In recent years I've been interested in the inorganic room temperature molten salt formed by cesium fluoride and hydrogen fluoride, in which liquid is formed with a ratio (at the eutectic point) in a ratio of 2.3 molecules of HF to one molecule of CsF.
Generally fluorine gas, an important industrial agent for a variety of reasons, is formed by electrolysis of a related system in which potassium fluoride KF, complexes similarly with HF. However this system requires significant heat to melt, thus raising the energy cost associated with the preparation of F2 gas.
This is discussed in a nice paper in the Journal of Fluorine Chemistry published in 2006: Cesium fluorohydrogenate, Cs(FH)2.3F (Journal of Fluorine Chemistry Volume 127, Issue 10, October 2006, Pages 1339-1343)
In the introduction the authors write:
It has been known that alkaline metal fluorides (MF, M = alkaline metal) form complex salts with HF [1,2]. These salts are composed of M+ cations and fluorohydrogenate ((FH)nF−, where n is an integer) anions. KF–HF system is used as an electrolyte for electrochemical synthesis of elemental fluorine and many studies have been made on its physical, chemical and electrochemical properties [3–5]. A similar application was also examined for CsF–HF system, although it was not applied to industrial electrolysis due to the high cost of CsF [2,6]. The advantage of CsF–HF system is its low melting point compared to KF–HF system which enables the electrolysis to be performed at lower temperatures to save energy and give broader choice of materials of electrolytic cells. According to the phase diagram [2], the CsF–HF system has a eutectic point below room temperature at the composition of CsF:HF of 1 to 2.3 (m.p. 16.9 °C), corresponding to the formula, Cs(FH)2.3F."
As I was poking around looking for this paper, I came across a paper by Neil Bartlett who is famous among chemists for his discovery, in 1962, that xenon, until then thought to be totally inert, could form compounds. Since 1962 many xenon compounds, most often fluorides but also oxides and other complex compounds, have been discovered, some by Bartlett himself.
The Barlett paper to which I refer, published just two years before his death in 2008 is this one:
Low temperature preparation and uses of potent oxidizers (Journal of Fluorine Chemistry Volume 127, Issue 10, October 2006, Pages 1285-1288)
Normally silver exhibits one oxidation state, 1+, although the 2+ state, analogous to that of its cogener copper, is well known.
Nickel's most common oxidation state is 2+, although a 3+ oxidation state is well known.
The Barlett chemistry is described in the graphic of his reactions found in the paper which may be seen by simply accessing the abstract. In this case, however, Barlett has prepared silver in the 3+ oxidation state, and nickel in the 4+ state, extremely unusual oxidation states.
These compounds, which are fluorine complexes are powerful oxidizing agents, and, as detailed in the paper, they are useful to prepare the hexafluorides of both rhodium and ruthenium (as well as platinum.)
Why is this important?
In less than 15 years the world supply of rhodium from ores, an important industrial catalyst and material with important technological implications, is expected be smaller than the supply available in used nuclear fuels. It is not clear that the element will be available from geological sources at economically recoverable levels at all in the next 20 to 50 years.
At that point it may become necessary to secure rhodium from used nuclear fuels, since that supply from ores will either have been depleted, or insufficient to meet demand for the metal.
Hexafluorides are known for 18 elements, and for all of them, the compounds are either gases or low boiling liquids. Historically used nuclear fuels have been recycled using complexes formed in solvents - the Purex process - which leaves a fair amount of chemical waste and other by products.
A far superior approach will be pyroprocessing including electrolytic recovery, perhaps in molten salts, including, but not limited to inorganic molten salts. (Organic room temperature molten salts - aka "ionic liquids" - are well know and vastly discussed compounds also of potential utility in the recovery of metals from complex mixtures like used nuclear fuels.)
Access to the hexafluorides of ruthenium and rhodium - both elements fairly large constituents of the fission products in used nuclear fuels - will allow for their recovery by simple distillation from the salts, a very clean process compared to extraction. In the case of rhodium in particular, although ruthenium is also an important and valuable element.
I note that uranium, neptunium, and plutonium, which are also valuable constituents of used nuclear fuels also form hexafluorides. The oxidation of americium to its highest state - AmF5 - also makes its separation from the lanthanide fission products and curium much easier to perform, although, regrettably, not as a gas.
Interesting and important implications I think, exciting chemistry to chemists of a certain type.
Have a nice Sunday afternoon.
A nice website put together by young engineers.
I love young engineers, the last best hope of humanity.
"About
This website was founded as a non-profit project, built entirely by a group of young engineers. Entire website is based on our own personal perspectives, and does not represent any views of any company in the energy industry.
Main purpose of this project is to help the public to learn some interesting and important information about the energy and about the nuclear energy. We realize that the basics in the nuclear physics do not belong to fundamental human knowledge and the term “nuclear” often evokes a feeling of something negative or even dangerous. We do not claim this or that opinion is the only opinion that is right. But it is noteworthy, that the vast majority of nuclear engineers, people who know what nuclear means, do not connect the term “nuclear” with anything negative or dangerous."
Nuclear Power for Everybody.
They have some very nice, nerdy quizzes.
Fast, accurate determination of cadmium contamination without expensive instrumentation.
According to the comprehensive survey of health risks to which I often refer for a qualitative assessment of environmental risks, A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010 (Lancet 2012; 380: 2224–60), occupational exposure to cadmium was killing 555 people per year as of 2010, up from 288 people per year in 1990.
The trend suggests that the the death toll may be higher as of 2017 than it was in 2010.
The annual death toll for cadmium exposure, of course, is much greater by two orders of magnitude than people killed by Fukushima, but nobody cares, since the solar industry, one of the largest users of cadmium, often in combination of two other highly toxic elements, selenium and/or tellurium, is "green," and nuclear energy, um, isn't. That's the common perception anyway, completely irrational, but common.
Twenty or thirty years from now, all of the "green" solar cells on this planet will be ready for landfills, although undoubtedly some inoperative examples of this "green" technology will just rot on the roofs on which they were installed, ultimately leaching their contents. Future generations, who are already doomed to pay for our generation's environmental ignorance anyway, will have a much worse risk from cadmium exposure than we do, since cadmium has been distributed to make distributed energy, a very, very, very, very bad idea, but one which is popular, completely irrational, but popular.
With this in mind, I found the following paper in the June issue of ACS Sustainable Chemistry and Engineering to be quite interesting:
ACS Sustainable Chem. Eng. 2017, 5, 4976−4981
The authors, Tianxiang Wu, Jiao Shan, and Zhanfang Ma, are Chinese, which is appropriate because much of the world's cadmium in mined in China. According to a study conducted at Nanjing University in 2007, 10% of the rice crops surveyed in six agricultural regions were contaminated with cadmium. (cf: Lancet 2013; 381: 2044–53)
Unfortunately, the best current approach to analyzing for cadmium contamination, is to use ICP/MS or ICP/MS/MS, instruments that are expensive to purchase and expensive to operate, usually employing a highly trained analytical chemist to run and interpret the device. This is especially true for complex matrices like soil, rice, and flowerbeds exposed to rain runoff from roofs with old "green" solar cells on them. If one is interested in the speciation of cadmium, one must also utilize a chromatographic system generally.
Wu, Shan and Ma's paper is involved with developing a simple, instrument free, and cheap way to detect and quantify cadmium without the use of expensive instrumentation.
First I'll refer to the author's introductory paragraph describing the hazards of cadmium exposure:
"
The chemistry of the colorometric test employed by the authors utilizes gold nanoparticles, and exploits the capability of gold cadmium alloys to catalyze certain reactions (reduction by sodium borohydride) that change the color of a dye toluidine blue O. Since the amount of gold is so small as to be invisible, the price of gold is trivial to the preparation of analytical kits.
The test is said to be as sensitive as electrochemical testing, having an LOD (limit of detection) of roughly 30 nM (nanomolar), not as sensitive as ICP/MS, but sufficient to identify concentrations of concern. The linear range of quantification is 30 - 480 nM, and in cases where the linear range is exceeded, quantification can be accomplished by serial dilution.
This sort of thing is likely to be important to future generations, especially as we are in an unending race to insure their impoverishment.
Interesting I think, and slightly encouraging since it will help to address an unavoidable bad situation for the current residents of China, who are enduring all sorts of things so Americans can "go green" and future generations who will need to clean up - to the extent they have resources to do so - our mess.
Enjoy the weekend.
Paper on the Equation of State for High Efficiency Supercritical Carbon Dioxide Driven Turbines.
I came across a very cool paper in a recent issue of one of my favorite scientific journals recently about one of the subjects that's been on my mind a lot recently, the physical chemistry of the dangerous fossil fuel waste carbon dioxide.
The paper is this one: Selection of a Proper Equation of State for the Modeling of a Supercritical CO2 Brayton Cycle: Consequences on the Process Design (Jaubert, et al, Ind. Eng. Chem. Res., 2017, 56 (23), pp 6841–6853)
The introductory paragraph from the paper describes better than I can why this paper is important:
If we accept a general working figure for the worldwide generation of electricity of 25,000 TWh per year this translates to about 90 exajoules (EJ) of energy per year of pure electricity, but not, it should be noted, the amount of energy consumed to generate electricity since the production of electricity always (from the second law of thermodynamics) will be less, generally much less, than 100% efficient. While modern combined cycle gas plants utilize both the Brayton cycle as referenced in the title of this paper coupled to a Rankine (steam) cycle can have efficiency values greater than 50%, most thermal plants, including most fossil and nuclear plants operate at roughly 30-35% efficiency.
Let's imagine for argument's sake that all of the 25,000 TWh (90 exajoules) of electricity were generated at 40% efficiency overall, which is probably not all that far from reality. Then the total energy expenditure on electricity generation would be on the order of 90EJ/.4 =225 exajoules, this out of a total energy demand (as of 2015) of 574 exajoules. Increasing the efficiency of by 5% to 90EJ/.45 = 200EJ would result in a total energy savings of 25 exajoules. To put this in perspective, 25 EJ is roughly 5 times as large as all the energy produced by all the so called "renewable energy" plants - solar and wind plants combined on the entire planet. These plants were constructed over 50 years of wild cheering for them and wishful thinking about them and the expenditure in just the last 10 years has been approximately two trillion dollars, with the result that climate change is accelerating, not decelerating.
Twenty five exajoules is also roughly equal to the thermal output of all of the world's nuclear plants, most of which operate on technology developed in the 1960's and were built over a 20 year period from roughly 1965 to 1985.
The author's raison d’être for producing the paper concerns the design of putative power plants which might utilize a carbon dioxide driven Brayton cycle.
The Brayton cycle, for those who do not know, is a power cycle in which the work (turning an turbine connected to a generator in a power plant) is performed without a phase change, i.e. the working fluid is a gas (or perhaps a supercritical fluid) without any liquid being involved. The most familiar example of a Brayton cycle device is a jet engine. In a jet engine, air is compressed and mixed with fuel - a vaporized dangerous fossil fuel in almost every case - and the fuel is ignited heating it and causing it to expand rapidly. In the jet engine, the exhaust pushes a transport device, generally an aircraft; in a power plant the exhaust expands against a turbine, driving its rotary motion.
We may contrast the Brayton cycle with the more common Rankine cycle, in which steam is generated from liquid boiling water (generally under pressure to raise the boiling point significantly beyond 100oC) until it reaches its boiling temperature at the pressure in the boiler, giving steam, and the steam expands against the turbine, driving it, after which it is cooled, and condensed back into a liquid, and returned to the boiler for reboiling.
A combined cycle device uses both cycles. Generally Brayton cycles are conducted at very high temperatures - they depend on so called "superalloys" often coated with refractory ceramics to keep the turbines and combustion systems from melting at these temperatures. The exhaust from the Brayton device is thus generally hot enough to boil water, and thus is available to drive a Rankine cycle. Most combined cycle plants utilize unsustainable dangerous natural gas as a fuel, but as I will point out below, there is no particular reason that this technology cannot be utilized in nuclear plants.
Every gas plant built and operated everywhere, by the way, no matter how high its efficiency level is a crime against all future generations. This includes power plants being built like the one proposed in Superior Wisconsin to help a power company "go renewable."
It's amusing to see people opposing gas lines because so called "renewables" are so wonderful. The entire renewable energy industry is nothing more than a fig leaf for the gas industry.
But I digress.
Returning to the motivation behind this fine paper though, the authors write in paragraphs following the first:
Existing studies have pointed out that the property change of CO2 near the critical point would result in a significant efficiency improvement (i.e., the compression work can be substantially decreased),5 along with a non-negligible influence in the turbo machinery design.6 However, the impact of the choice of the thermodynamic model used to evaluate the properties ofCO2 has not drawn attention. As an example, Dostal et al.,5Kato et al.,7 Zhang et al.,8 Jeong et al.,9 Lee et al.,10 and Serranoet al.11 all selected the Span−Wagner EoS without justifying their choice. As a second example, the study performed by Clarke et al.12 used a law of corresponding states and highlighted that a small change of the physical properties like the heat capacity and density affect significantly the heat-exchanger design and performance.
Regarding the chemical and energy industries, Whiting et al.13 pointed out that surprisingly, there are only a few studies devoted to the analysis of uncertainty associated with thermodynamic models despite their pivotal role for process design and simulation."
One of the chief thermodynamic penalties paid in Brayton cycles is the energy consumed by the compressor, and the energy, in turn, required to run the compressor is very much a function of the thermodynamic state of the gas, said states being determined by an "equation of state." The state of the gas also plays important roles in turbine design, especially material design, and of course, in heat transfer devices.
There are many examples of gas equations of state, the most familiar to most high school science and freshmen college students being the famous "ideal gas law," PV = nRT. The law is just a loose approximation, not very useful for meeting the requirements of sophisticated engineering devices or for that matter, chemical plants. The trade off between simplicity and accuracy is a difficult one to navigate, and increasingly sophisticated gas laws have been developed over the last century, beginning with the "cubic" laws, the Van der Waal's gas law, then the Reddich Kwong law, it's refined version, the Soave-Reddich-Kwong followed by the widely used "Peng-Robinson" gas law. The latter law was designed to give a method which could generate supporting constants from readily available physically measurable properties of the gas, specifically its critical pressure, critical temperature and a factor known as the "acentric factor," nominally a function of molecular geometry. (The critical pressure is the pressure at which the liquid phase and gas phase become indistinguishable, and the critical temperature is the temperature at which that occurs.)
A very accurate law has been developed for a specific gas, the dangerous fossil fuel waste carbon dioxide, has been developed; this is the Span Wagner equation referred to in the text above. The derivation of this law is an intellectual tour de force; it relies on analysis of the Helmholtz energy, which is the "free energy" equation at constant volume corresponding to the more familiar Gibbs free energy at constant pressure.
It is described in a classic paper, Span and Wagner Journal of Physical and Chemical Reference Data 25, 1509 (1996). Google Scholar has it being cited just over 3000 times; in comparison the Peng Robinson equation paper Ind. Eng. Chem. Fundamen., 1976, 15 (1), pp 59–64 - also a tour de force which is utilized to describe many gases and thus has broader utility - is one of the most cited papers of all time, with just over 8,900 citations.
The Span-Wagner equation in an earlier time - it includes a number of transcendental functions - would have been too complicated for much utility inasmuch it would eat vast amounts of computer time, faster more powerful computers have made it practical to use.
The authors of the paper cited at the beginning of this discussion are, in effect, complaining that people utilize the Span Wagner law without justifying it; the purpose of the paper is to justify it, by comparing five other gas laws and their effect on the design of important power plant components.
The authors write:
.• Cubic EoS: the Peng−Robinson EoS (PR) using theclassical Soave alpha function; the Peng−Robinson EoScombined with the Boston−Mathias alpha function(PR-BM), an alternative version of the PR EoS; and theSoave−Redlich−Kwong EoS (SRK) using the Soave alphafunction
• Virial-type EoS: the Lee−Kesler−Plöcker EoS (LKP) andthe Benedict−Webb−Rubin modified by Starling andNushiumi (BWRS)
• Helmholtz-type EoS: the Span−Wagner (SW) EoS."
The authors conclude that the use of the Span Wagner equation, their "reference equation" is in fact justified in making design decisions for power plants. Their goal again was not to disprove the acceptability of the Span Wagner equation, but rather to prove in a formalized sense that the decision to use it - given the power of modern computers - is acceptable.
However, there is a huge caveat: The equation is optimized in a range from the triple point temperature (the temperature at which gas, liquid and solid can coexist) and the corresponding triple point pressure to a limited high temperature, 1100K.
Span and Wagner report the triple point values with high precision as:
"Tt Triple-point temperature: Tt=(216.592± 0.002) K;
Pt Triple-point pressure: Pt=(0.517 95± 0.000 10) MPa;"
Note that the triple point temperature is on the absolute Kelvin scale, and that this temperature is very cold, -56.558oC, but it is the upper bound that is of concern, 1100K, which translates to a relatively modest 827oC, which is, in theory, hot enough to run a power plant on a combined cycle basis, but still not as high as one might go to achieve broader goals in the elimination of the use of dangerous fossil fuels, that is to completely and totally phase them out, something future generations will be required to do, even if our generation has been too silly and too distracted to do it ourselves.
(As for the triple point pressure, 0.517 MPa is about five times atmospheric pressure. The Span Wagner equation is valid at pressures that are 8000 times higher than atmospheric pressure.)
There are many reasons to explore Brayton Cycles that operate at significantly higher temperatures than 1100 K, particularly if one wishes to thermochemically split water into hydrogen and oxygen. There are many known thermochemical cycles, all of which are significantly more efficient than water electrolysis. Hydrogen and (pure) oxygen are useful chemical intermediates that would in theory allow for the conversion of any source of high temperature gas or supercritical fluid into hydrogen and oxygen at fairly high thermodynamic efficiency.
Probably the best known thermochemical cycle is the sulfur-iodine thermochemical cycle, first advanced many years ago by General Atomics (largely because the patent on it had expired) for use with their high temperature helium Brayton type reactor, the HTGC type reactor. Thousands of papers relating to the sulfur iodine thermochemical cycle are typically published in a given year; for the link I just picked one more or less at random.
When I was a kid, and first heard of the sulfur iodine cycle, I was very excited by it, but in fact, this cycle is more problematic than many other available potential thermochemical water splitting cycles. I've lost my taste for it, because of the issues of corrosion and mass transfer; in particular the production of 1 gram of hydrogen requires the transfer of 128 grams of hydrogen iodide, and in any case, iodine is expensive, even if it is designed to be continuously recycled.
In fact, part of one potential thermochemical cycle is already practiced on a huge industrial scale, in particular the "water gas" reaction by which 99% of the world's current hydrogen production (which is important for the critical industrial enterprise of making ammonia). The water gas reaction is as follows:
CO + H2O ↔ H2 + CO2.
In this case, as industrially practiced in these energetically irresponsible times, the carbon monoxide is formed by the partial oxidation of methane, the main constituent of dangerous natural gas.
It is, however, very possible to make carbon monoxide by reducing carbon dioxide. Analogously to water splitting thermochemical cycles there, are thermochemical carbon dioxide splitting cycles that carry out the reactions as well that carry out the following reaction:
2 CO2 ↔ O2 + 2 CO.
The sum of these reactions, after doubling the first, is simply yet another water splitting reaction:
2H2O ↔ 2H2 + O2
Thus By coupling this reaction with the water gas reaction we can see that combining these two reactions, one already performed on an industrial scale, we would have in effect, a thermochemical water splitting reaction that potentially would be far less corrosive than the sulfur iodine cycle.
Here’s an example of a thermochemical cycle that works to split either water or carbon dioxide, which I pulled out of my files at random:
Ceria as a Thermochemical Reaction Medium for Selectively Generating Syngas or Methane from H2O and CO2 (ChemSusChem 2009, 2, 735 – 739)
Pretty much any compound now obtained from petroleum can be made with access to syngas, which is a mixture of hydrogen and carbon monoxide as well as, in some cases carbon dioxide.
These cycles generally require temperatures significantly higher than 1100K, usually hundreds of degrees higher, although the cited paper indicates a reaction temperature that is only slightly higher, 1173K. It is notable that there are now many kinds of materials that have been designed that selectively permeate gases, including oxygen, hydrogen (the metal palladium has long been known to conduct hydrogen) and carbon dioxide as well as many other gases. Advances in materials science processing have also been known to produce materials that are biphasic, containing two types of conducting polymers.
Here's an example of a paper that contains two types of oxygen conducting polymers.
Chang et al, Journal of Membrane Science 322 (2008) 429–435
It is therefore possible to imagine advanced materials that allow the permeation of very hot carbon dioxide into them and conduct oxygen out themselves in one direction and carbon monoxide in another. Therefore one can imagine adding a third combined cycle aspect to a supercritical carbon dioxide reactor, a thermochemical portion that allows the production of motor fuels (to the extent we require and accept them), Brayton cycle electricity, and finally, in the final cooling phase, Rankine cycle electricity. We're certainly not there yet by any means, but it certainly is not beyond feasibility, but my readings in materials science suggest that these ideas are not entirely out to lunch.
My son, who is graduating this evening from high school with high honors, is planning to major in materials science engineering in college, with a long term goal of being admitted to a good or great materials science graduate program.. I hope I will be able to stick some of these thoughts into his head, should he decide that his old man actually isn't too stupid to engage.
In any case, to return to the original point, we will certainly need, in order to design these types of devices, advanced equations of state. In order to construct a ternary combined cycle as just imagined, we would need, at the very least, to extend the Span Wagner equation to higher temperatures.
It's worth doing. As I've noted over the years in many different places, the uranium and thorium already mined are sufficient to provide all of humanity's energy needs for centuries if they are utilized in breeding nuclear fuel cycles. This would mean no fracking, no oil platforms, no coal mines, far less excessive and toxic silicon processing, and in fact, no uranium or thorium mining, at least for centuries to come.
Advances in material design, notably in refractory ceramics (including some that are machinable, the so called “MAX phases”) now suggest that such high temperatures will be accessible. It is worth noting that some of the highest melting temperature ceramics include actinide based ceramics that potentially could serve as both structural and fuel elements, examples include thorium oxide (which has been used industrially as a high temperature crucible material), uranium nitride, thorium, uranium and plutonium carbides, etc.
Interesting, if esoteric.
Have a nice evening.
A "Maxwell's Demon" For Carbon Dioxide Capture?
Recently I had the pleasure of acquiring a wonderful monograph Molecules at Work, subtitled "Selfassembly, Nanomaterials, Molecular Machinery"
I can't tell you that I've actually read it yet, but it certainly promises to be interesting when and if I get around finally to reading it, or at least sections of it. The part I can't wait to read is the part about molecular machines which are exactly what they sound like, since molecules (or small groups) of molecules that perform machine like activities.
Probably the best known molecular machine occurs naturally, the enzyme ATP synthase, which is located in the mitochondria of eucaryotic cells. This molecule rotates in such a way as to "force" a phosphate moiety onto ADP to make ATP, the latter molecule functioning as the "energy currency" of the cells of all higher organisms. The driving force for this activity is a proton gradient, basically an electric field. Fluorescent labels have been attached ATP synthase and the motion of the enzyme has actually been filmed microscopically.
Before the actual discovery of molecular machines, the great physicist James Maxwell posited one in (what came to be known as) a "thought experiment." This was "Maxwell's Demon", the "Demon" being a tiny being on a molecular scale who could actually work to violate the 2nd Law of Thermodynamics (a law that has apparently always been unpopular with certain kinds of people like, um, say, Amory Lovins, and people who think that everything can be saved by ever more Rube Goldberg types of energy storage systems. Energy storage, because of the 2nd law, must always waste energy, but no matter trying to educate people on that score.)
The Demon was posited to examine a question in the then arising kinetic/statistical theory of gases which states that heat may be thought of as a function of the average speed of molecules in a gas. The hotter the gas, the faster the average speed. Of course since the speed is an average it follows that some of the molecules are moving fast and others slower but the temperature observed is the canonical ensemble of all the molecules. The way the "Demon" was imagined to violate the second law (as well as the "zeroth law"
The "problem" of the demon has now been solved by appeal to information theory, where the demon is required to consume energy to make the decisions, thus he is nothing more than a heat pump, a device (like a refrigerator or air conditioner) that consumes energy in order to produce a temperature gradient. Thus the 2nd law is "safe," and, as Einstein had it - it will always be safe - because it's a fundamental law of nature that cannot be overruled.
Today, nevertheless scientists are now actually building new kinds of molecular machines, these on the scale of the Maxwell's imagined "Demon." I came across a fun one in a journal I've added to my reading list, Chemistry of Materials. (My son is starting a Materials Science Engineering education, and I must admit I'm vicariously thrilled at the prospect of such an exciting intellectual pursuit.) This "demon" opens and shuts trap doors to let carbon dioxide molecules in and out of its nanoscale (yoctoliter) containers.
Here is a link to the paper, which may be behind a firewall, but can be accessed in a good university or college library:
Azobenzene Guest Molecules as Light-Switchable CO2 Valves in an Ultrathin UiO-67 Membrane (Knebel et al, Chem. Mater., 2017, 29 (7), pp 3111–3117)
In recent years there have been major advances in organometallic chemistry relevant to materials science, highly structured molecules with huge surface areas, known as "MOF's" or Metal Organic Frameworks. These, which are loosely related but not really the same as naturally occurring zeolites are useful as catalyst supports since they have a huge surface area per unit volume, and also for gas separations, gas storage, the removal of toxic elements and molecules from dilute solutions, etc.
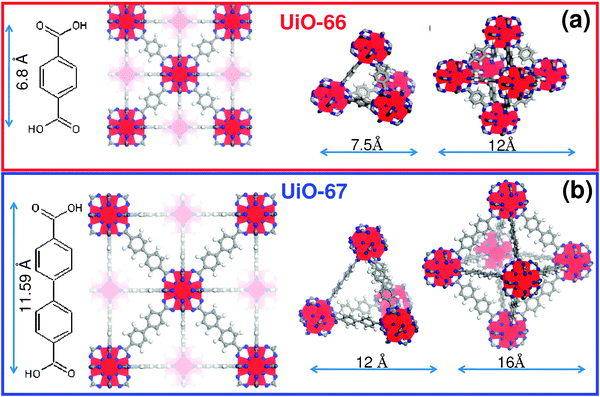
UiO-67 - the trivial name refers to its discovery at the University of Oslo in Norway - is an array of zirconium oxide complexes supported in a framework of 4,4' biphenyl dicarboxylates:
Some text from the paper's introduction:
"
A description of a gas separation experiment in which a mixture of carbon dioxide and hydrogen is separated by use of the synthetic molecular machine:
"
Note that this "demon" consumes energy to operate; it is not, of course, a kind of perpetual motion machine.
Biphenyls, as highly conjugated molecules are sometimes utilized in some electrically conducting organic polymers, and I suppose that some people, looking at this system could make all kinds of leaps about artificial photosynthesis, leading to all kinds of wishful thinking about a solar powered world.
The solar scheme for addressing climate change is, however, a miserable and expensive failure that will never be as clean, as safe, or as sustainable as nuclear energy. This said, it will fall to future generations to deal with the consequences of our tremendous failure to be responsible, and these kinds of molecular machines might well have practical applications for the incredible task with which we have left all future generations, that is, to clean up our mess. The separation of carbon dioxide from dilute matrices, air, or better, seawater, will be an essential tool for any hope they may have for success. It may not be this molecular machine, or any molecular machine at all, but it is wise to explore these avenues until their potential utility is understood.
Esoteric, I think, but interesting.
Have a nice day tomorrow.
Unstable Solutions to Thermodynamic Differential Algebraic Equations From Equation Solvers.
All of humanity's efforts to address climate change have failed, and thus it will fall to the up coming generations to reverse it, this with reduced access to rapidly depleting resources including not only endangered chemical elements, but of course, access to things which very much depend on the environment, specifically food and water.
This is a far more challenging engineering task than it would have been to eliminate the use of dangerous fossil fuels but I've comforted myself about the way we've screwed future generations by noting that they will have access to some tools we lacked, in particularly computational capabilities of which my generation could only dream.
For a number of years now, I've been interested in the alternative fuel DME, dimethyl ether, not just its synthesis and use as a fuel, but also in some properties connected with its transport. DME is an easily liquified gas, and as such, I think - though I haven't seen it widely discussed - may have utilization in certain heat transfer applications, including long distance heat transport and heat storage (utilizing recent advances in materials science.) The use as a working fluid in heat transfer, either as a heat pump fluid or a refrigerant - each the reverse of the other - involves phase changes, as does transport in pipelines.
Although I have very little experience with computer modeling of thermodynamic equations of state, I decided to poke around in some of the usual journals I read to see what people are saying about this capability and I came across some interesting papers that are relevant to my daydreams about transporting and storing heat in a DME media.
For example there is this one about pipelines - CO2 pipelines - involved in a scheme to address climate change that will not work, carbon capture and sequestration: Solution of the Span-Wagner Equation of State Using a Density Energy State Function for Fluid-Dynamic Simulation of Carbon Dioxide (Ind. Eng. Chem. Res., 2012, 51 (2), pp 1006–1014) which contains the following fun text:
"
Carbon capture and storage (CCS) has been proposed as a strategy to reduce carbon emissions to the atmosphere. An important part of the CCS chain is transportation, either in pipelines or in tanks (on boats, vehicles or trains), from a capture point to a storage site. To ensure efficiency and safety in these operations, it is important to have accurate simulation tools to control the processes, and also to perform risk analysis. For instance, an issue with transportation of the supercritical substance (CO2 is transported at high pressure, in order to minimize pipeline dimensions) is crack propagation. If a pipeline ruptures, the crack may propagate along the pipe, depending on the speed of the crack, relative to the speed of the expansion wave inside the pipe.1, 2 To simulate this process, an accurate model for the flow inside the pipe is needed. Another example is the global trade of CO2 quotas, which has also been suggested as a means to reduce CO2 emissions.3 From an economic perspective, it is important to describe the CO2 properties accurately in order to determine the amount of CO2 stored and transported..
The authors refer the reader to the original Span-Wagner equation utilized "to describe the CO2 properties accurately" and make this note:
We see that the equation contains a total of 51 terms, many of which include logarithms and exponentials. This means that this is an expensive function to evaluate, and efficient numerical methods are critical to obtain satisfactory run times for dynamic simulations.
But, one needs to be very careful about how one performs these calculations, as I learned reading another related paper from the same scientific organization, this one:
Time Efficient Solution of Phase Equilibria in Dynamic and Distributed Systems with Differential Algebraic Equation Solvers (Ind. Eng. Chem. Res., 2013, 52 (5), pp 2130–2140)
This paper contains this mildly disturbing text:
"
The most common approach to solve systems with phase equilibria is to perform the calculations in inner loops, separated from the higher-level modeling. This method will be referred to as the traditional methodology in the rest of this work. The advantage is that algorithms tailored for robust flash calculations can be applied. A disadvantage is that the approach leads to nested iterations loops. Numerical noise is when the fluctuations of internal variables due to limited accuracy are comparable to the predefined solution tolerance, which can give an unstable solution."
The authors explore the use of the commercial MATLAB program and Fortran to reduce the calculation time.
The issue here is that one can get kind of disconnected to the nuts and bolts of equation solvers, and in fact, face "butterfly effects" in which the calculations go awry.
I've given my son, starting an engineering program this fall, the task of programming the solutions to the Soave-Reddich-Kwong equation and the Peng Robinson equations in both Mathematica and Matlab, the former because I bought it for him and the latter because the engineering universities all seem to use it.
I need to make him aware of these kinds of risks when he goes to a deeper level than pure play.
Esoteric, but interesting.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,513

