NNadir
NNadir's JournalSince Republicans care a great deal more about fetuses than children or adults, maybe this paper...
...can be utilized to convince them to give a rat's ass insisting that we really shouldn't care more about haircuts and bars than people.
Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection (David Baud, MD, PhD1; Gilbert Greub, MD, PhD2; Guillaume Favre, MD1; Journal of the American Medical Association (JAMA), published on line April 30, 2020)
This woman's medical outcome is of course a tragedy, but Republicans seem to have a more narrow definition of tragedy than real human beings do, and perhaps this might drill through their thick heads.
The Global Disinformation Index:
I'm on the mailing list for the AAAS blog, and there was a chat today on the AAAS blog with Dr. Danny Rodgers of the "GDI," the Global Disinformation Index.
It advertises a "neutral" approach to statistically rate the credibility of information.
Dr. Rodgers biographical info (from the website) is this:
From the "live chat" a participant asked:
Since so much of this disinformation seems to be disseminated via social media channels, can you recommend accessible sources for accurate information that folks could be referred to when responding to false claims?
Any success stories you might share for those if us struggling to reach people with accurate info?
Thanks!
...to which Dr. Rodgers replied:
Remember, too, re platforms like Facebook: on the internet, when the product is free, YOU'RE the product.
The site, in their "research heading" describes their methodology:
The GDI rating system takes a ‘whole-of-site’ approach to understand the risk of a news site disinforming its readers. Advertisers and ad tech companies can use the GDI ratings to shape brand safety decisions about where their ad spends end up.
[link:https://disinformationindex.org/wp-content/uploads/2019/12/GDI_Index-Methodology_Report_Dec2019.pdf|Rating
Disinformation Risk: The GDI Methodology]
A brief excerpt:
...The ‘Content’ pillar contains indicators that assess different elements of news articles published on a specific domain, including their credibility, sensationalism, neutrality and impartiality. As for all of the pillars, each of these indicators was chosen to identify and measure a specifc disinformation signal or fag (see Appendix A). How a domain’s content is presented and covered is an important indicator of the disinformation risk of the domain. Some of the more pernicious forms of disinformation occur when news domains present a variety of straight and accurate news with a few maliciously and purposefully inaccurate stories in order to gain and manipulate users’ trust…
...The ‘Operations’ pillar assesses the underlying policies and rules that domains abide by to establish trust and reliability in the quality of the news being published. The integrity of a news organisation and its site is a good indication of whether checks and balances are in place to prevent or lower the risk of disinformation from appearing on a site...
...The ‘Context’ pillar assesses the overall credibility and reliability of news-related information provided by a specifc domain. The overall conduct of a site can go beyond a sample of content and the operational policies in place. It relates to how the news domain is viewed: the overall perceived trustworthiness of the site. The disinformation fags assessed in this pillar are related to credibility, trustworthiness, conficts of interest and biasedness. As these signals are not easily measurable by analysts, this pillar and the questions in it are assessed by country-level experts working on media and related issues...
I have only superficially evaluated this Index, but the concept strikes me as interesting and worthy of some more study to understand their approach.
We certainly do live in the world of disinformation, driven not just by Rupert Murdoch and Fox "News" but by many others as well. A successful system like this might well help raise a level of critical thinking.
Clinical Improvement in Covid Remdesivir Double Blind Study Is Not Statistically Significant.
This morning I'm seeing noise that Remdesvir is working in treating Covid patients.
Yesterday, the results of the first randomized clinical trial were published.
The results study were published yesterday in Lancet: Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial.
(The publication is open sourced; anyone can read it.)
Here is the summary of the findings, for convenience:
A small benefit was observed, however it cannot be ruled out that this benefit derived from chance.
I wouldn't call the results "clear evidence" that this drug is a worthy treatment, unless I'm missing something.
We May Have Hit The Annual Maximum CO2 Observatory at Mauna Loa Unusually Early This Year.
As I've indicated many times before, somewhat obsessively I keep a spreadsheet of the weekly data at the Mauna Loa Carbon Dioxide Observatory, which I use to do calculations to record the dying of our atmosphere, a triumph of fear, dogma and ignorance that did not have to be, but nonetheless our atmosphere is dying.
In my last post touching on this point, this one, I wrote:
The record set then, for the week beginning April 5, 2020, was 416.45 ppm, 3.78 ppm higher than the same week of the previous year.
If we look at the data from this week, and from last week (416,27 ppm) as well, we will see that both are lower than the figure observed on April 5.
Here is this week's data:
Weekly average CO2 at Mauna Loa
Week beginning on April 19, 2020: 415.88 ppm
Weekly value from 1 year ago: 413.71 ppm
Weekly value from 10 years ago: 393.25 ppm
Last updated: April 26, 2020
The difference for this week's reading compared to last year of the same week, 2.17 ppm is very close to the average of these readings in the 21st century, which is 2.16 ppm. The average for these readings for the 20th century, going back to 1975 was 1.54 ppm in comparison to the same week of the previous years.
I took a look at the data going back 44 years, to discover the earliest dates of annual peaks, and found that there were six times in the last 30 years in which the peak occurred in April, eight such years overall. April 5, if this date holds for this year, is the second earliest date, marking the beginning of a peak week, at which the peak has ever occurred. The earliest peak date was a peak set in April 4, 1999. It is effectively same week of the current observation owing to calendar fluctuations. The previous year, 1998, was until 2016, the record setting year for carbon dioxide increases, 2.93 ppm over 1997. Two readings in this decade, 2015 and 2016, superseded that record, both coming in at 2.99 ppm over the previous year. 2018 reached 2.83 ppm.
In 1998, the peak was reached on the week of May 24, 1998, when it reached the (then) disturbing level of 369.94.
In 1980, the peak arrived on the week of June, 1, 1980, the latest date recorded for such a peak. In that week, the peak value was 340.61 ppm. Except for some then obscure academics, including some who may have been aware of Arrhenius's prediction of climate change in the 19th century, no one probably thought much about that number.
Why, if it holds, the peak came so early this year is unknown. One may surmise that it is an effect of the severe restrictions placed on the car CULTure brought on by the Covid-19 catastrophe. We have been living with reduced access to our precious cars, as people always deign to inform me, without which we cannot live. Even with restrictions on our cars, it does seem to me that many of us are still alive, but perhaps that will change.
Another possibility is that as a result of climate change, spring is arriving earlier in the Northern Hemisphere, as carbon dioxide readings continue each year to decline through September before beginning to rise again. However a survey of the data shows that an April peak was observed as early as 1986, on the week of April 27, 1986, when the concentration of carbon dioxide was 350.71.
It is therefore difficult to say what this early peak occurred this year, only that it did.
At the Mauna Loa CO2 Observatory website the following is written about the effect of Covid-19 on climate change, which is a fair statement:
There have been many inquiries whether we can see in our CO2 measurements at Mauna Loa and elsewhere the slowdown in CO2 emissions from the burning of fossil fuels. That drop in emissions needs to be large enough to stand out from natural CO2 variability caused by how plants and soils respond to seasonal and annual variations of temperature, humidity, soil moisture, etc. These natural variations are large, and so far the "missing" emissions do not stand out, but we may see them as the year progresses. Here is an example: If emissions are lower by 25%, then we would expect the monthly mean CO2 for March at Mauna Loa to be lower by about 0.2 ppm. When we look at many years of the difference between February and March we expect March to be higher by 0.74 ppm, but the year-to-year variability (one standard deviation) of the difference is 0.40 ppm. This year the difference is 0.40 ppm, or 0.33 below average, but last year it was 0.52 ppm below average.
Most of the emissions come from urban areas, so that it may be easier to see the effect downwind of cities, although also in that case they need to stand out from natural variations. Only measurements of carbon-14 in CO2 would enable us to cleanly separate fossil sources of CO2 from ecosystem sources and sinks regardless of how variable the latter are.
If any of these figures disturb you, don't worry, be happy. Cruise around the internet to find any of zillions of web pages talking all about how great solar energy is and how it will save the day.
It hasn't saved the day; it isn't saving the day; and it won't save the day, but it's the thought that counts.
Have a pleasant Sunday evening; stay healthy; stay safe.
The Greater, Ignored Plague, Disease and Water Use and Storage In India.
The paper I'll discuss in this post is this one: Household Water Storage Management, Hygiene Practices, and Associated Drinking Water Quality in Rural India (Sarah L. McGuinness,* Joanne O’Toole, S. Fiona Barker, Andrew B. Forbes, Thomas B. Boving, Asha Giriyan, Kavita Patil, Fraddry D’Souza, Ramkrishna Vhaval, Allen C. Cheng, and Karin Leder, Environ. Sci. Technol. 2020, 54, 8, 5062-5070).
Echolalia is the practice of repeating yourself or others without meaning, often thought as a sign of psychiatric symptoms, senility, for example, among old people.
And then, of course, there is a process of repeating one's self for emphasis, as when nothing meaningful gets through until it is, or nearly is, too late. (Think of the then old man Winston Churchill whining that the Nazis might be, um, bad people in the 1930's, when he was largely ignored.)
As the poet Amari Baraka put it in one of my favorite pieces of verse:
And what I have learned
of it, to repeat, repeated
as a day will repeat
its color, the tired sounds
run off its bones
...as a day will repeat its color, the tired sounds run off its bones...
I am old and the sounds that run off my bones are tired sounds...and I repeat myself, and whether what I write is senile echolalia or a warning issued in the hope it is not entirely too late is not for me to judge, but for any interested reader to judge, if there are interested readers: I am infinitely more obscure than Winston Churchill was in 1938 and everything I say, whether it is echolalia or otherwise, will die with me, obscure.
One of the tired sounds I repeat is that so called "renewable energy" is a grotesque failure if the reason for embracing it was in any way connected to addressing climate change. This should be clear from the simple reality that climate change has not been addressed. It is getting worse, not better.
In 1976, a guy named Amory Lovins, then 29 years old, wrote this piece of unreferenced appalling drivel on the subject of Energy and the Environment: Energy Strategy: The Road Not Taken? in a social science journal, Foreign Affairs. The claim made in this intellectually appalling text, that so called "renewable energy" and energy conservation would save the world has become "The Road Most Taken" in attempting to address climate change: Many hundreds of thousands of scientific papers have been written on the subject of so called "renewable energy," the research funded by rich sources of grants, and as some people on this website never tire of pointing out, wind farms and solar cells are enormously popular, if entirely ineffective, bourgeois conceits.
Since 1976, Amory Lovins, who is now 73 years old has been engaged in echolalia, having become famous for writing the aforementioned piece of appalling drivel, declaring himself the Chief "Scientist" of an ignorance factory called the "Rocky Mountain Institute," inviting people to tour, for a fee, his obscene (but very energy efficient) McMansion in the very, very, very upscale community of Snowmass, Colorado, just down the road from the hip, hip, hip, hip skiing community of the "Stars," Aspen. Amory Lovins' echolalia has been to repeat, "as the day repeats its colors," the hip, hip, hip mantra that energy conservation and so called renewable energy will save the world. He makes a lot of money doing this, but he still asks you to contribute money to support his "genius."
In my opinion, he was senile - or at least uneducated - at the age of 29, since the subject of conservation and energy had been discussed in predictive terms that actually involved accurate predictions, based on data as opposed to handwaving, by the British economist William Stanley Jevons. His famous theory is known as "Jevons Paradox" which states, simply that the more efficiently a resource is utilized, the more of that resource will be utilized.
I repeat myself, "as the day will repeat its color..."
Here is what I wrote about Amory Lovins theology back in 2014, since I am not really a modern liberal who thinks that liberalism involves supporting huge wind farms in pristine wilderness, including offshore wilderness, and electric cars laced with cobalt mined by enslaved children in Congo, but I am an old liberal, inasmuch as I care about poverty, like Lyndon Johnson, like FDR and his remarkable wife, Eleanor Roosevelt:
Current Energy Demand; Ethical Energy Demand; Depleted Uranium and the Centuries to Come
..."as the day will repeat its color"...
Here is another thing I repeat frequently:
The amount of money "invested" in so called "renewable energy" in the period between 2004 and 2018 is over 3.036 trillion dollars; dominated by solar and wind which soaked up 2.774 trillion dollars.
Source: UNEP/Bloomberg Global Investment in Renewable Energy, 2019
Then I point out, "as the day will repeat its color" that this expenditure on so called "renewable energy" took place on a planet where more than 2 billion people lack access to even primitive sanitation using this link from the organization, recently maligned by the US's Chief Ignoramus, the World Health Organization:
Lack of sanitation for 2.4 billion people is undermining health improvements
To step outside of echolalia briefly this 2015 link has an update: WHO and UNICEF launch updated estimates for water, sanitation and hygiene
Then I point out, "...as the day will repeat its color..." that these billions of dollars that have been spent on so called "renewable energy," exceeds the entire GDP of India, a nation with 1.35 billion people in it, for everything Indians do, eat, educate themselves, build shelters, factories farms and drink.
This, I guess, brings me to the paper referenced at the outset of this repetitious diatribe. From the introductory text:
The optimal approach to ensuring safely managed drinking water is to provide treated piped supplies directly to households.4,6−9 However, while access to piped water supplies in LMICs is increasing,3 financial and logistical barriers to their widespread implementation remain, and existing piped supplies are often intermittent, compromising water quality and availability.6,10 Most previous trials of low-cost water treatment solutions for LMICs have evaluated in-home pointof- use interventions such as chlorine products and filters, but these have recognized limitations, including the need for high user adherence to consistent sustained behavior change.7,11 Few studies have evaluated alternative approaches, such as community-level treated water supplies, in part because randomized trials of such interventions are challenging to design and conduct.12 The delivery of treated water supplies or treatment of existing supplies reduces the requirement for user behavior change, increasing the potential for sustained and consistent access to clean water.13,14 However, fecal contamination of water can still occur during collection, transport, or subsequent storage of water within the household.8,9 Household water storage is a common practice in settings where on-premises supplies are unavailable or intermittent.9,15...
The authors toured rural Indian villages, where they conducted surveys and then sampled the drinking water of the people they surveyed and ran microbiological tests for fecal pathogens. Some excerpts of their procedures:
...Water Sampling Visits. During the pretrial consent process, permission was sought for collection of household water samples at subsequent study visits. Among a random sample of households (165 per village per survey round), drinking water was collected by a designated water sampling team. Participants were asked “If you or your child wanted a drink of water right now, where would you get the water from?” Permission was then requested to view the relevant stored water container or water source, and participants were asked to collect drinking water in the usual manner. This water sample was obtained from the householder and decanted into a sterile 120 mL sample bottle containing sodium thiosulfate sufficient to neutralize 15 mg/L chlorine in 100 mL of the sample (Colilert, IDEXX Laboratories, USA) by a member of the water sampling team using an aseptic technique. The water sampling team documented the storage vessel location...
The documentation also included details about how the water was stored, covered or uncovered, and how it was delivered, by ladle, for example. The scale of the documentation can be seen in table 1 in the paper, which reports on the findings of homes with contaminated water:

Some graphical results from the paper:

The caption:
Some sample pictures of storage systems observed:

The caption:
The findings of risks in the sampled water:

The caption:
Some longitudinal data:

The caption:
The data speaks for itself, doesn't it?
...data...
..."as the day will repeat its color"...
As for that 29 year old kid referenced above who was not familiar with Jevons in 1976 but was declared a "genius" by some people who also hadn't read Jevons, and of course, by himself, he is now 73 years old; he was born in 1947, the age of the "baby boomer."
I am a baby boomer, profoundly ashamed of my generation and the way we so facilely latched on to what can only be described as silliness and delusion.
Our mantras about so called "renewable energy" did not work; they are not working; they will not work.
To my knowledge, there isn't data on the carbon dioxide concentrations in 1947, when Lovins was born, but the data at the Mauna Loa carbon dioxide observatory goes back to 1959, when the concentration was 315.97 ppm.
Less than 20 years later, the world would embrace the "conservation and renewable energy" Mantra Lovins proposed.
Here is the latest weekly data on the concentration of carbon dioxide in the planetary atmosphere as of this writing:
Weekly value from 1 year ago: 413.63 ppm
Weekly value from 10 years ago: 3 92.85 ppm
Last updated: April 25, 2020
..."as the day will repeat its color"...
I do not know what world energy demand was in 1947 when Amory Lovins, but I do know what it was in 1973, just three years before he wrote his now famous drivel about so called "renewable energy" and conservation. It was 253 Exajoules, as reported in the 2011 World Energy Outlook.
..."as the day will repeat its color"...
In the most recent year that we have data, 2018, world energy demand was less than an Exajoule short of 600 Exajoules.
By the way, world wide, energy efficiency, has risen dramatically since 1976.
At times, we tend to focus on the income disparity of the United States, which is now sure to grow, as the right wing through inattention and manipulation has brought on what is sure to be a world wide economic depression.
..."as the day will repeat its color"...
As scientist who has echolalia dating back to 1976 uninfluenced by data is not actually a "scientist" at all, even if his title is "Chief Scientist" of an organization like say, "The Rocky Mountain Institute." Arguably, he is nothing more than the leader of a cult.
Hopefully, in this time of cults, political, economic, spiritual and otherwise, this emerging worldwide economic depression doesn't end like the last one did, with the worst war in human history.
One way to prevent that in my perhaps naive opinion is to consider that those people in India are human beings, just like people in Africa, in Mexico, in Canada, and, indeed the United States, that they matter, as all human beings worthy of being called human beings matter, because there is no justifiable reason that these people in India and in similar places should...
...while we obliviously wax romantic about our Tesla electric cars, wind turbines, and efficient refrigerators.
I might mention that one of the best and well known ways to sterilize water, and in fact, to remove some very dangerous pollutants is to irradiate it with ionizing radiation.
As the day will repeat its color, even in a time of the rising celebration of ignorance, I continue to believe, even as my life approaches its end, "the tired sounds running off my bones" that intelligence and decency can yet prevail.
I wish you health and safety in this spring weekend, and some measure, within the limits of our time, peace and happiness.
'A disaster': Roche CEO's verdict on some COVID-19 antibody tests
'A disaster': Roche CEO's verdict on some COVID-19 antibody testsRoche is one of the leading companies in the world in the diagnostic field, and manufacturers equipment that meets the CLIA regulations for medical testing, a "go to" company.
I am not involved in any way in diagnostics, but I worked closely with Roche scientists in the early days of the AIDS crisis when people were dying without access to drugs. There was a quality problem with an intermediate that just bordered on the regulatory limits. The decision was not an easy call to make at all; again lives hung in the balance. People would die without that drug, quite literally. I however was very impressed by the professionalism and seriousness with which the Roche Scientific team considered the issue; and was impressed with how hard they worked to resolve the issue in a way that, um, saved human beings.
I mention this because one might hear in connection with this article, all kinds of stuff about big bad corporate people squashing little guys. I personally don't take it that way at all. I suspect the Roche CEO is being honest. Yes, he has skin in the game, but it is inaccurate and in fact, intellectually dishonest, to think that all Pharma executives are "in it for the money."
That's all I'll say on that score.
Roche’s (ROG.S) diagnostics business has moved out of the shadow of its main medicines unit during the pandemic, as the Swiss pharma giant confirmed its 2020 sales and profit outlook amid rising demand for COVID-19 testing...
...An erroneous false-positive result could lead to the mistaken conclusion that someone has immunity. In developing its test, Schwan said, Roche scrutinised some existing products for reliability before rejecting them.
“It’s a disaster. These tests are not worth anything, or have very little use,” Schwan told reporters on a conference call. “Some of these companies, I tell you, this is ethically very questionable to get out with this stuff.”
Schwan said there were about 100 such tests on offer, including finger-prick assays that offer a quick result. The Basel-based company declined to specify which rival tests it had studied, but said it was not referring to tests from established testing companies...
...Schwan did not release figures for its test’s “specificity”, or how many false-positives can be expected, but promised it would be reliable because Roche had successfully found the antibody produced by the body after exposure to the novel virus.
“This is really what matters,” he said. “Every kind of amateur could produce an antibody test. The two of us could do it overnight in the garage. That’s not the problem.”
“The question is, does it really work? And for that, you have to do testing and validation,” he added.
"From below, from basements, from cellars, from sewers they rose..."
As I've remarked a few times in this space, in this lockdown I'm translating Camus' La Peste (The Plague) from French into English, appropriate in these times.
I found this paragraph - presaging the coming human plague, where he describes massive numbers of rats crawling of from under the city dying - despite being grotesque, to be quite evocative and profoundly metaphorical, and in some ways, relevant, more than half a century after Camus's work was published, to the "rise" of Repuplicans in Washington.
My translation:
The original French:
The more things change, the more they stay the same, n'est pas?.
A Nice Scientific Review Article on the Destruction of Persistant Perfluoroorganic Pollutants.
The paper I'll discuss in this post is this one: Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review (Junkui Cui, Panpan Gao, and Yang Deng, Environmental Science & Technology 2020 54 (7), 3752-3766). Although EST is primarily a journal that reports on original research, it also features some viewpoint, policy and, as in this case, review articles.
Review articles, as every scientist knows, refer to other papers in the scientific literature to generate the authors' consensus on the state of a particular area of research.
The situation with respect to the widespread, and highly intractable PFAS (perfluoroalkyl substances) contamination in the environment is a very hot topic because these synthetic molecules were and are widely used. The most familiar perfluoroalkyl substance that most people have in their homes, often in multiple settings, is Teflon. Teflon when overheated can degrade into volatile perflouro compounds, which are known to kill, for example, caged birds in people's homes. It is generally not fatal to human beings of course, but being non-fatal does not imply that ingestion of perfluoro compounds is good for you. There's a lot of evidence in fact that it's not good for you. Perfluorocompounds have also been widely used in many other types of commercial products, spray on fabric protectors for furniture (although these products, including Scotch Guard, are no longer available), fire fighting foams, where massive amounts were sprayed on fires to put them out, electronic devices, and hydrogen fuel cells. As a result, they are widely distributed in water, ground, and biological flesh, including human biological flesh, where they are thought to represent a growing risk. I will briefly refer to the text of the review article & a few interesting graphics here and then comment on a few papers I found referenced in the review, as well as a few I came across in references in the references.
I am very interested in this topic because I am very interested in the utilization of radiation resources, because it turns out that radiation is a very potent tool for addressing this otherwise intractable problem.
The introductory text of the review gives a nice overview of the problem:
However, concerns on PFAS have gradually been growing due to their prevalence, mobility, persistence, bioaccumulation, and adverse health effects.(2,5−9) Many PFAS have been demonstrated to bioaccumulate, can bind to blood proteins, and have long half-lives in humans. Human PFAS exposure is linked to cancer, obesity, elevated cholesterol, immune suppression, and endocrine disruption.(10−12) Various PFAS, particularly perfluorooctanoic acid (PFOA) and perfluorooctanesulfonate (PFOS), have been frequently identified in surface freshwater,(13−15) groundwater,(7,16−19) drinking water,(20−22) and landfill leachate.(23−27) The emerging anthropogenic chemicals are challenging water and wastewater treatment, water reclamation, site remediation, and landfill leachate disposal because of a lack of effective, efficient, and practical treatment technologies. Meanwhile, a tremendous pressure potentially derives from the regulatory determination. In 2016, US Environmental Protection Agency (EPA) issued a Lifetime Health Advisory (LHA) for PFOA and PFOS at 70 ng/L.(21) Presently, US EPA is moving forward with the maximum contaminant level (MCL) process for PFOA, PFOS, and probably more PFAS chemicals in drinking water.(28) The pursuit of public health and potential regulatory pressure require the water industry to stay current and proactive for advancing innovative PFAS treatment technologies. Among very few technically effective PFAS treatment methods, advanced reduction processes (ARPs) have recently emerged as a promising option.
Note that the remark about the EPA is historic, and refers to a historical time when there was actually a President of the United States who did not hate his country and its citizens, who actually cared about their health and in fact whether they lived or died.
Obviously this situation is no longer obtained. The country is being run by a group of very nasty and ignorant people known as Republicans, who couldn't care less about whether Americans are healthy, safe or live or die, so long as their donors and themselves can make money and worship an intellectually and morally paralytic as part of a twisted and sick religion. Apparently in their "faith" fetuses, and of course, guns, are more important than living breathing people.
This however, is the science forum, not the religion forum, so excuse the aside.
A covalent chemical bond that exhibits cylindrical symmetry around the bond axis and exhibits high electron density in the region between the atoms in the bond is known as a "σ bond." Among σ bonds, because of the high electronegativity of fluorine - the highest in the periodic table - the carbon fluorine bond is the third strongest, after the boron fluorine bond and the silicon fluorine bond. (The nitrogen fluorine bond is also very strong, NF3, a greenhouse gas, is now found in the atmosphere and its concentration, like many other fluorine containing gases including hydrofluorocarbons, sulfur hexafluoride and related compounds, is rising.)
To break a carbon fluoride bond, therefore requires significant energy.
If we take the bond dissociation energy of the carbon fluorine bond from this table, 536 kJ/mole, and then calculate using Avogadro's number, the unit electric charge, and Planck's constant the frequency of light that has the energy to dissociate this bond, we find that the frequency is around 1.3 PHz. (PetaHertz), or 1.3 X 10^(15) Hz, corresponding to a wavelength of around 220 nm. This is in the ultraviolet region, specifically the UVC region, which is the wavelength of UV radiation is used to sterilize objects, is carcinogenic since it also breaks bonds in skin, and is filtered out largely by the ozone layer. Without such filtering terrestrial life could not exist.
Period.
However, simply breaking this bond with light at this wavelength does not necessarily prevent the bond from reforming, and thus it is not the case that simply irradiating PFAS, even with UV radiation, destroys them. This is also observed in the case of the breaking of chlorine or bromine carbon bonds in the upper atmosphere, and accounts for the fact that CFC's have persisted a long time, being effectively catalytic owing to recombination of the original chlorine (or bromine) radicals after they go through happily destroying oodles and oodles of ozone molecules in chain reactions. Relatively rare reactions do terminate these chain reactions in such a way as to lead to the destruction of CFCs them over time. These reactions are rare in the upper atmosphere, because water is very dilute there: It is the reaction with radicals derived from water that cause the decomposition of CFC's, with the chlorine or bromine ending us as either hydrochloric or hydrobromic acids. Happily these destructive CFC or Brominated carbons are faster than is the case for HFC's, which - unless something is done to change the situation - they will persist for tens of thousands, even hundreds of thousands of years. The lifetime of most CFC's is thought to be decades or hundreds of years.
PFAS in water and soil are not exposed to UV radiation except in industrial settings, thus they will persist for a very, very, very long time.
There have been a huge number of papers in the scientific literature on the subject of PFAS, which is a good thing, since it is a very serious matter, many on destroying them by various chemical means, and but the paper here focuses on a particular approach - it may be the best approach, which is reductive processes, what is called in the paper "advanced" reductive processes. A reducing agent is anything that gives up an electron readily. Alkali metals, which are very reactive, are all reducing agents, cesium metal being the strongest among them. When dissolved in liquid ammonia, pure ammonia and not the familiar household aqueous solution, these metals actually produce one of the most powerful reducing agents, the solvated electron.
It is also possible to create solvated electrons in water, using - this is unsurprising - UV (or higher energy) radiation which causes water to dissociate into hydrogen radicals and hydroxide radicals, the latter being one of the most powerful oxidizing agents.
This graphic from the article is a schematic cartoon showing the way that solvated electrons interact with PFAS to decompose them in multiple steps. The graphic will also show, besides free electron, the present of sulfur and iodine species which, as discussed in the review article, are actually designed to generate free electrons. To wit, from the text:
The caret ( ^ ) here is used to designate superscripts which are no longer available in the DU editor.
The iodine and sulfur species can react with the generated (destructive) PFAS radicals to form iodinated or sulfinated species which are far less persistent, and far easier to destroy than PFAS themselves, often by biological or readily available chemical means, and thus do not represent quite the same risk as the PFAS.
This is shown in the graphic from the paper:
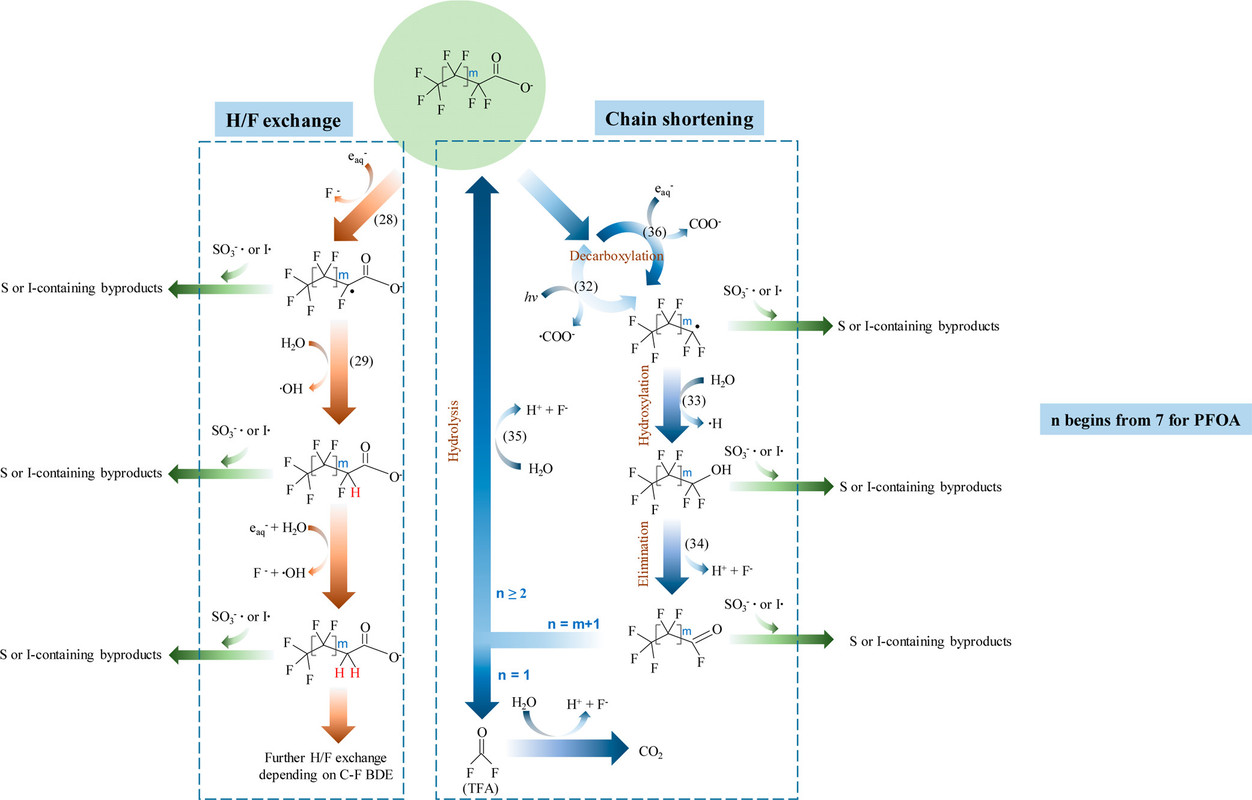
The caption:
PFOA is a very common PFAS, found in many products. PFOA is the abbreviation of perfluorooctanoic acid. The destruction of PFOA can proceed by both pathways, the one on the left and the one on the right.
Note that there is an error in this diagram. The molecule on the very bottom of the right identified as "TFA" which generally stands for the common reagent trifluoroacetic acid, is not TFA. It is fluorophosgene, a very toxic molecule which thankfully has a very short half-life in water, especially in basic solution. (The common fun reagent known as "phosgene" - used in the first world war as a war gas - is chlorophosgene.)
Fluorophosgene can also be produced from the persistent greenhouse gas tetrafluoromethane, which is a side product of the aluminum industry when carbon electrodes made from petroleum coke are oxidized by electrochemically generated fluorine gas in the Hall process. In considering the radiochemical destruction of this gas, I was surprised to learn a few years back that a precursor of fluorophosgene, trifluoromethanol is surprisingly long lived; I'd assumed that its decomposition to fluorophosgene was very fast, but apparently I was wrong. I believe that trifluoromethanol can be isolated although it would definitely be unpleasant stuff to be around, since ultimately it will decompose to fluorophosgene.
Note that all of these pathways lead to the generation of HF, highly corrosive hydrofluoric acid. However, in these pathways, the concentration of this acid (or its gaseous form) happily remains relatively low. Both HF and fluorophosgene are conveniently destroyed by bases; calcium hydroxide being one of the best, since it produces insoluble calcium fluoride. I performed this neutralization many times myself when I was a kid; it works great.
Another graphic shows a similar set of pathways for the decomposition for another major component of PFOS, which is also abbreviated "PFOS" but where the "OS" means "octanoyl sulfonate" rather than "organic substances." Perfluorooctanoyl sulfonate is another widely distributed product found, like PFOA, in ground water, river water, and living things.
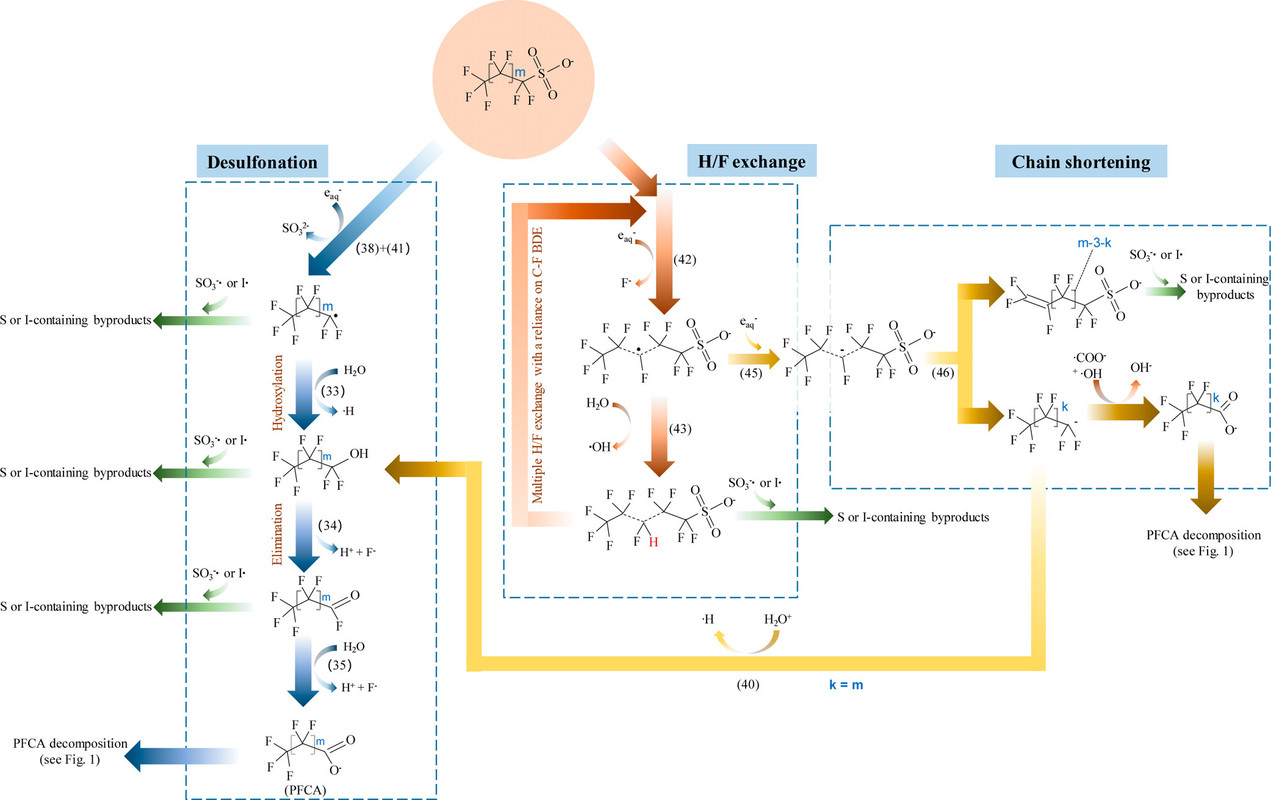
The caption:
In this case they have avoided the possible confusion about the meaning of the PFOS abbreviation by referring calling them perfluorosulfonic acids.
Reference 68 in this paper is this one:
The Hydrated Electron (Herbert and Koons, Annual Review of Physical Chemistry Vol. 68:447-472 (2017)).
I referenced in another post in this space, a quick sidelight referring because of an interesting historical note in it, but the thread turned annoying in such a way that my sense of humor deteriorated, something easy to do.
Nevertheless, the paper has an interesting graphic that shows the pathway of generation of solvated electrons:
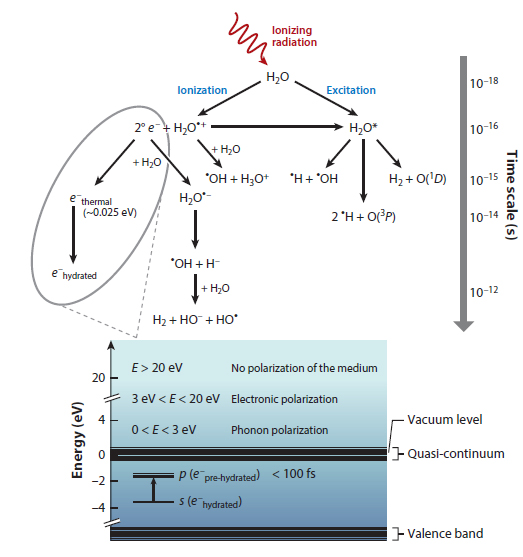
The caption:
In the context here, the radiation is UV radiation, although it need not be, higher energy radiation will work, a subject to which I'll return below.
Reference 47 in this paper just cited is, by the way, this one: Precursors of Solvated Electrons in Radiobiological Physics and Chemistry (Elahe Alizadeh and Léon Sanche, Chem. Rev. 2012, 112, 5578−5602)
When I went to open it, I noticed that the particular issue of Chemical Reviews, which is about 8 years old, had a number of review papers on solvated electrons, and I poked around in a few of them. A paper not specifically about solvated electrons per se, but certainly touching on them concerned the degradation of Nalfion membranes in hydrogen fuel cells, which are often represented as being "green" although Nalfion is a perfluorinated polymer and there is nothing, despite much thermodynamically illiterate nonsense one can read everywhere, including in the scientific literature, about hydrogen that is green. Almost all of the hydrogen produced on an industrial scale is made by utilization of the dangerous fossil fuel dangerous natural gas.
Nevertheless, these fuel cells are commercial, but are utilized mostly as back up power for critical systems in power outages, cell phone towers, medical facilities and the like.)
The paper on their degradation is this one:
Fuel Cell Perfluorinated Sulfonic Acid Membrane Degradation Correlating Accelerated Stress Testing and Lifetime (Marianne P. Rodgers,* Leonard J. Bonville, H. Russell Kunz, Darlene K. Slattery, and James M. Fenton, Chem. Rev. 2012, 112, 6075−6103). The discussion in the paper refers to the decomposition products of Nalfion, one of which is hexaflouroacetone. Another is hydrofluoric acid. Hexafluoroacetone is often utilized in analytical chemistry in the HPLC and LC/MS/MS of nucleic acids and therapeutics derived from nucleic acids. When it is, it requires special precautions on the part of the laboratory science since hexafluoracetone is an extremely toxic compound, if I recall correctly is a powerful hepatotoxin. I am aware of many labs that have refused to work with the compound, although obviously some do.
Nevertheless, hydrogen fuel cells are "green." So I've heard.
By the way, people who are always prattling on about "green cars," an oxymoron if there ever was one since there is no way to make the car CULTure sustainable, often talk about hydrogen fuel cell cars, and in fact, this is a topic of the Chemical Review paper just linked, how to make fuel cells for cars.
Returning to the subject of the more common perfluorocompounds addressed in the opening post, a point made in some of the references and in other papers I've read independently on this topic is that the solvated electron, a reducing agent, is very much the main species responsible for the degradation of PFOAs. Interestingly solvated electrons are produced in the same pathway as the powerful oxidizing agent, the hydroxide radical, as mentioned above.
Obviously the recombination of these species can lead to lower effectiveness for radiation's effects on PFOA. There is a way to shift the equilibrium toward solvated electrons as opposed to OH and H radicals, and this is via the use pH.
This is discussed in this paper: Effect of initial solution pH on photo-induced reductive decomposition of perfluorooctanoic acid (Yan Qu, Chao-Jie Zhang ⇑, Pei Chen, Qi Zhou, Wei-Xian Zhang, Chemosphere 107 (2014) 218–223. The authors find a profound effect of pH, with the hydrated electron surviving longer at high pH. The UV wavelength they were utilizing was 254 nm, somewhat longer than the 223 nm wavelength I calculated above, although this wavelength was generalized to a "typical" C-F bond and not necessarily the wavelength in say, PFOA. The solvated electrons are generated via an iodide intermediate.
The authors propose the following rationale for the effect of pH that they find:
Eqn 8:

Electronic repulsion between the negatively charged hydroxide and the charged solvated electron and differences in the hydration spheres of the various species prevent similar addition to the OH- ion to quench electrons by formation of the hydroxide radical which in any case, were it to exist, be doubly charged.
It should be said that in this particular setting, the authors noted the presence of perfluorinated gases such as CF4, C2F6, etc, so there's that. It also must be said that they needed to rigorously exclude oxygen from the system.
The importance of hydration spheres on kinetics involving solvated electrons is discussed in this paper: Hydrogen Forms in Water by Proton Transfer to a Distorted Electron (Jungwirth, et al., J. Phys. Chem. B 2010, 114, 2, 915-920)
Japanese authors have noted that pH has little effect in the presence of organic molecules in the system: Factors influencing UV photodecomposition of perfluorooctanoic acid in water (Giri et al., Chemical Engineering Journal 180 (2012) 197– 203) An interesting point made in this paper is the influence of wavelength. Standard UV lamps (Hg lamps) radiate at the 254 nm mid UV range. Diode array instruments, widely used in analytical chemistry can reach down to around 200 nm, but the intensity of these beams is not required to be very high, and there is a point at which glass absorbs UV. The authors in this paper used a special kind of synthetic glass that was designed to transmit the higher energy shorter wavelength 185 nm light.
This led to a dramatic change in the performance of the system in degradation as shown:

The caption:
Of course, there is radiation that is much more energetic than 185 nm wavelength energy, specifically X-rays and gamma rays. These radiations can produce very high energy electrons either through photoelectric or the related Auger process besides directly scissioning carbon fluorine bonds.
A review article going back some 15 years ago on the subject of electrons in water, explicitly covers very high energy electrons, whereas the other papers discussed in this thread discusses moderately high energy electrons, those in the 5-6 eV range, the UV range. In all cases, the papers discuss the thermalization of electrons at these energy, thermalization being the process by which their energy is reduced to levels associated with the kinetic energy of molecules to those associated with ambient temperatures.
This paper is here: Role of Water in Electron-Initiated Processes and Radical Chemistry: Issues and Scientific Advances (Chem. Rev. 2005, 105, 355-389) It's written by a consortium of scientists representing a fair percentage of the major radiation laboratories in the United States, both National Labs and Academic Institutions.
The point here is that highly energetic electrons vastly enrich the number of electrons, and thus the rate of decomposition of analytes reacting with the rich array of reactions initiated by these electrons.
Figure 1 from this paper shows that it all begins when ionizing radiation produces electrons, the more energetic the electrons will be.
Figure 1:
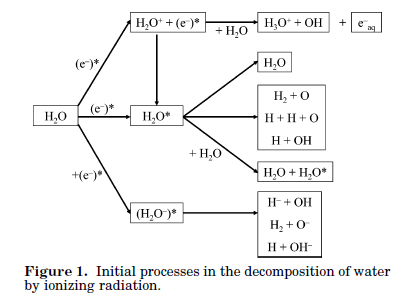
The caption:
The richness of the chemistry of water in a radiation field is given by a table from this paper:

Of course many of the species here are highly reactive in their own right, and many would serve to degrade many contaminants in dangerously degraded water, including, but not limited to PFOSs.
Cesium hydroxide is the strongest base known in aqueous solution and I have gone so far as to contemplate a solution of highly radioactive cesium hydroxide in which the radioactive cesium was added directly, as the hydroxide, to severely contaminated water. In the PFAS case, there are many examples of groundwater of this type, particularly in the upper midwest. However, the amount of the most readily available highly radioactive isotope of cesium, Cs-137, is relatively small. A crude calculation on my part, based on a simplified solution to the Bateman equation, the simplification justified by the low neutron capture cross section of Cs-137 and the fact that nuclear power has been producing about 28 exajoules of energy per year suggests that on the entire planet there is only 200 - 300 metric tons of this isotope, a tiny amount when compared to the sheer volume of highly contaminated water on this planet.
Moreover, the cesium-137 available in used nuclear fuel is not isotopically pure, it is contaminated by the non-radioactive isotope Cs-133 (cesium's naturally occurring isotope which is also a fission product), very small amounts of cesium-134, a short lived isotope formed (half-life 2.06 years) by neutron capture in Cs-133, cesium-135, a very long lived radioactive isotope whose long half-life limits its use as a radiation source, and cesium-137.
A French paper some years back which utilized some elegant approaches to separate cesium isotopes from their non-radioactive barium daughters, gave some insight to the relative proportions of these isotopes in used nuclear fuel, by dissolving a used nuclear fuel pellet in nitric acid and using an analytical technique known as ICP/MS to measure the proportions of cesium isotopes in them, barium free, a neat trick. That paper is here: Cs–Ba separation using N2O as a reactant gas in a Multiple Collector-Inductively Coupled Plasma Mass Spectrometer collision-reaction cell: Application to the measurements of Cs isotopes in spent nuclear fuel samples (Granet et al., Spectrochimica Acta Part B 63 (2008) 1309–1314).
Here is a mass spectrum showing the cesium isotope distribution in freshly isolated nuclear fuel:

The caption:
There is no information about the cooling period this fuel pellet underwent, but it reasonable to assume that it wasn't all that long. Consider a used nuclear fuel that we might have in New Jersey, fuel removed from the Oyster Creek Nuclear Reactor in 1971, two years after the reactor came on line. (It closed last year, regrettably.) This fuel is 59 years old. The half-life of cesium-137 is 30.08 years. This means that only 25.6% of the original cesium-137 is still radioactive and that if the fuel's distribution looked similar to that in the French graph, the Cs-137 peak is only 1/4 the size it was back then. This means that the radioactivity is diluted significantly.
In recent years, I've come back to an idea that I abandoned when I knew far less about nuclear engineering than I know now, which is to put cesium salts into certain chambers in a nuclear reactor to conduct certain kinds of heat transfer tasks coupled with neutronics control tasks. The non-radioactive isotope of cesium, Cs-133, has more and stronger neutron capture cross sections in the epithermal region than any of the radioactive isotopes, meaning that it is readily possible to increase the radioactivity of "old" decayed cesium to make it highly radioactive because of the short half-life (and high energy) of the Cs-134 isotope to assist residual Cs-137 that has decayed even as much as 1971 Oyster Creek fuel.
Here is the neutron capture spectrum over the fission neutron range:

Even in the happy case where we produced all of our energy by nuclear means, we would only be producing less than 300 tons of fuel per year, the new accumulations being reduced each subsequent year as we approach ever closer to the Bateman equilibrium limit.
Nevertheless, the seriousness of the PFAS contamination levels on this planet which have hardly arrested, suggests we ought to consider something along these lines, assuming that we can get dumb guys and gals to reject their very dangerous fear and ignorance about radiation.
This is a long post, but I had some fun writing it, so I hope no one minds.
I wish you a safe and healthy week, and some pleasure from "making the best of it."
Jeeze, the doctor is only thirty...
So. as my lock down exercise, during an epidemic I'm translating Camus's La Peste ("The Plague" ) to see if I can revive my French language skills. This is the tale of Dr. Rieux, who we learned earlier is just 30 years old and who is relating the story of an outbreak of the plague in the city of Oran in the 1940s. I'm about halfway through the first chapter and I came across this exchange between the doctor and a journalist who wants to investigate the living conditions of the Arabs in (then) French colonial Algeria.
Here's an excerpt of my translation:
“I only grant information without restrictions. I cannot, therefore, support your investigation.”
“This is the position of Saint Juste,” said the journalist with a smile.
Rieux said, without raising his voice, that he knew nothing of that, only that this was the language of a man tired of the world in which he lived, having had, all the same, a taste of his contemporaries, having decided for his part to refuse the injustices and the concessions. Rambert, his neck on his shoulder, looked at the doctor.
“I believe I understand you,” he said finally, getting up.
Only the French can be that cynical at 30; they're better than we are. We have to be at least 55 to get there.
I'm only giggling a little bit because years ago, a friend and I used to perform this little amusing shtick in which we would pretend to be 1940's French intellectuals engaging in trop serieux absurdist ennui.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,512