NNadir
NNadir's Journal...this isn't a Russian poem, this is not somewhere else but here, our country moving closer...
What Kind of Times Are These?and the old revolutionary road breaks off into shadows
near a meeting-house abandoned by the persecuted
who disappeared into those shadows.
I've walked there picking mushrooms at the edge of dread, but don't be fooled
this isn't a Russian poem, this is not somewhere else but here,
our country moving closer to its own truth and dread,
its own ways of making people disappear.
I won't tell you where the place is, the dark mesh of the woods
meeting the unmarked strip of light—
ghost-ridden crossroads, leafmold paradise:
I know already who wants to buy it, sell it, make it disappear.
And I won't tell you where it is, so why do I tell you
anything? Because you still listen, because in times like these
to have you listen at all, it's necessary
to talk about trees.
-Adrienne Rich (1929–2012)
In a way, it's wonderful that she did not have to live to see this.
There is young cowboy...
?t=236New Alkaline-Earth Metal Fluoroiodates Exhibiting Large Birefringence and Short Ultraviolet Cutoff.
The paper I'll discuss in this post is this one: New Alkaline-Earth Metal Fluoroiodates Exhibiting Large Birefringence and Short Ultraviolet Cutoff Edge with Highly Polarizable (IO3F)2– Units (Minqiang Gai,§ Tinghao Tong,§ Ying Wang,§ Zhihua Yang, and Shilie Pan, Chem. Mater. 2020, 32, 13, 5723–5728)
Organohalide contamination of the air, hydrosphere and land is a very serious environmental issue. From my perspective, the best and least demanding approach to ameliorating this long term problem is high energy radiation.
The highest energy radiation readily available on Earth is gamma radiation, however in most matrices this high energy radiation is down converted to lower forms of energy, x-rays and high energy UV. The ability to control and direct, that is to focus, this high energy radiation is more problematic than it is with visible light, and thus materials that have the ability to refract UV radiation is certainly of interest.
This is why this paper caught my eye.
I won't spend a lot of time discussing it, but just offer a brief excerpt and a few pictures to give a feel for the paper, which is, in any case, relatively short.
...In this contribution, we reported one iodate Sr(IO3)2 (SIO) and two fluoroiodate compounds SrI2O5F2 (SIOF) and Ba(IO2F2)2 (BIOF). Among these compounds, SIOF and BIOF are the first reported cases in alkaline-earth metal fluoroiodates. It is noted that the (IO3F)2– units in fluoroiodates have not yet been reported. Interestingly, compared with the reported fluoroiodates, SIOF shows a large birefringence (cal. 0.203 at 532 nm) and its birefringence is about twice than that of SIO and BIOF. SIO, SIOF, and BIOF have short UV cutoff edges (255, 250, 230 nm) as well as good thermal stability. The mechanism of large birefringence source in SIOF and BIOF was discussed systematically...
Birefringent materials are materials that refract light - in this case UV light - differently - that is to a different degreen depending on orientation of the material with respect to beam path.
A few more comments from the paper about these properties:
For most of my life, I've just thought of the fluoroiodates as potentially relevant materials to certain reprocessing schemes for used nuclear fuel and as, well, interesting curiosities. This paper points out I've been missing, overlooking, some things.
Some pictures from the text:

The caption:

The caption:

The caption:
The conclusion:
None of this means very much I suppose in the grand scheme of things, but I reflect on things of this nature thus: I often rail against what my generation has done to all future generations with terror and disgust. Irrespective of the fact that history will not forgive us, as some slight redeeming facet is that the world is left with all these tiny punctilios of knowledge that may be sign posts for the future to do what can be done to restore the world.
Being able to focus and move UV radiation certainly is something that may be of value.
I trust you are having a pleasant and safe summer evening in these very difficult times.
Rosalind Franklin was so much more than the 'wronged heroine' of DNA
An editorial in the current issue of Nature:
Rosalind Franklin was so much more than the ‘wronged heroine’ of DNA
It's open sourced, but some excerpts:
As one of the twentieth century’s pre-eminent scientists, Franklin’s work has benefited all of humanity. The one-hundredth anniversary of her birth this month is prompting much reflection on her career and research contributions, not least Franklin’s catalytic role in unravelling the structure of DNA.
She is best known for an X-ray diffraction image that she and her graduate student Raymond Gosling published in 19531, which was key to the determination of the DNA double helix.
But Franklin’s remarkable work on DNA amounts to a fraction of her record and legacy. She was a tireless investigator of nature’s secrets, and worked across biology, chemistry and physics, with a focus on research that mattered to society. She made important advances in the science of coal and carbon, and she became an expert in the study of viruses that cause plant and human diseases. In essence, it is because of Franklin, her collaborators and successors, that today’s researchers are able to use tools such as DNA sequencing and X-ray crystallography to investigate viruses such as SARS-CoV-2...
...Franklin wanted to understand the porosity of coal, mainly to learn how to make it burn more efficiently. But, as Patricia Fara, a historian of science at the University of Cambridge, UK, points out, the porosity of coal was also a key factor in the effectiveness of Second World War gas masks, which contained activated-charcoal filters. As such, Franklin indirectly aided in the design of the personal protective equipment of her day...
...From coal, Franklin moved on to the study of viruses, which would fascinate her for the remainder of her life. During the 1950s, she spent five productive years at Birkbeck College in London using her X-ray skills to determine the structure of RNA in the tobacco mosaic virus (TMV), which attacks plants and destroys tobacco crops. The virus was discovered in the 1890s...
...With the structure of TMV resolved, Franklin set out to study other plant viruses blighting important agricultural crops, including the potato, turnip, tomato and pea. Then, in 1957, she pivoted again to begin studying the virus that causes polio, which is structurally similar to the turnip yellow mosaic virus. At the time, polio was a feared communicable disease. It has since been mostly eradicated, although cases linger in Pakistan and Afghanistan...
...In 1956, she was diagnosed with ovarian cancer, and she died two years later at the age of just 37. Her collaborators Aaron Klug and John Finch published the poliovirus structure the following year, dedicating the paper to her memory4. Klug would go on to be awarded the 1982 Nobel Prize in Chemistry for his work on elucidating the structure of viruses.
A broader story than the one you usually hear.
Desymmetrization of difluoromethylene groups by C-F bond activation
The paper I'll discuss in this paper is this one: Desymmetrization of difluoromethylene groups by C–F bond activation (Hartwig et al., Nature volume 583,548–553 (2020))
Whether it's known in the general public or not, the carbon fluorine bond, one of the most stable bonds known, is one of the most troubling issues in the environment; a subject about which I've written in this space previously.
Although the use of PFOS, PFOA and related compounds have been largely banned around the world, except in countries run by right wing morons who hate science and the environment, the situation is far from being resolved. There are, for example, technological efforts, such as the ill advised and environmentally dubious efforts to store energy to correct to address the issue that makes so called "renewable energy" environmentally, economically, and thermodynamically unacceptable and unsustainable. The much discussed approach to energy storage, and one that has been commercialized is the fuel cell, many of which have fluoropolymeric solid electrolytes. Some people are so thoughtless and rote that they thing hydrogen is a "clean fuel." It isn't. It's a thermodynamic and environmental nightmare, at least as it is currently made, and might be made in the quixotic adventure to make so called "renewable energy" matter.
Anyway.
I have thought a great deal about fluorine carbon bonds in my long life; particularly as I dig deeper into environmental issues, but this paper is more relevant to my initial interest in them, since they are, owing to their strong electron withdrawing nature coupled with an atomic radius close to that of hydrogen, very important in medicinal chemistry. Here's a nice review on the topic:
Applications of Fluorine in Medicinal Chemistry (Gillis et al., J. Med. Chem. 2015, 58, 21, 8315–8359)
Of course, in the "every problem is a nail if you only have a hammer" fashion, my feel for the means to address the flourine carbon bond problem in the environment is high energy radiation, given my fondness for the wonderful properties of used nuclear fuels - the materials that people with poor imaginations have named so called "nuclear waste" but anything that involves the activation of carbon flourine bonds captures my eye.
This one involves making chiral molecules from difluoromethylenes. It's pretty cool.
From the text:
Figure 1:
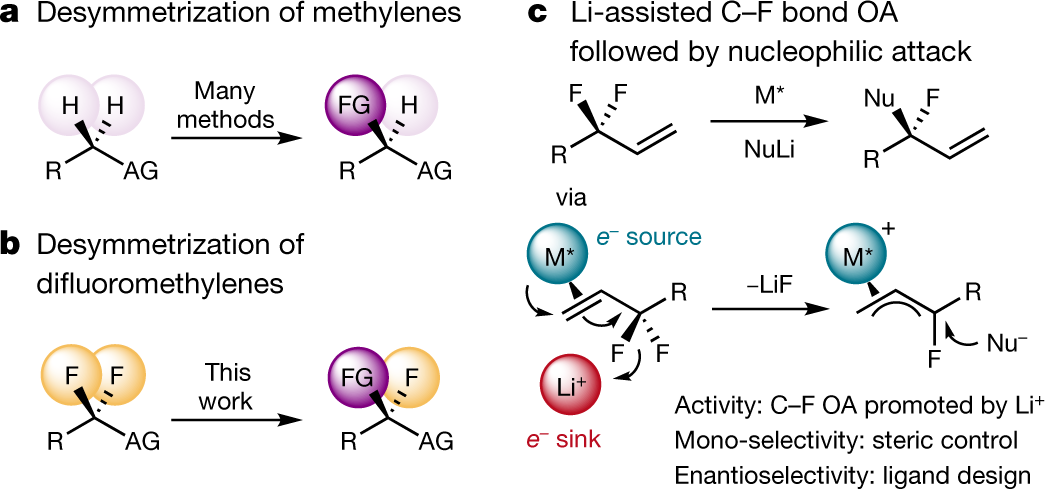
The caption:
The authors discuss their thinking further and then discuss their approach to this novel chemistry:
Iridium is, one should note, a very expensive and rare metal; its cogener, rhodium is also important in asymmetric chemistry, supplies of rhodium can be maintained by isolation from used nuclear fuel although it is fairly rare (and thus expensive) when obtained by mining.
Phosphoamidites are well known, and very important in synthetic nucleic acid chemistry; and are in general no big deal.
Their catalyst structure is shown in this figure:
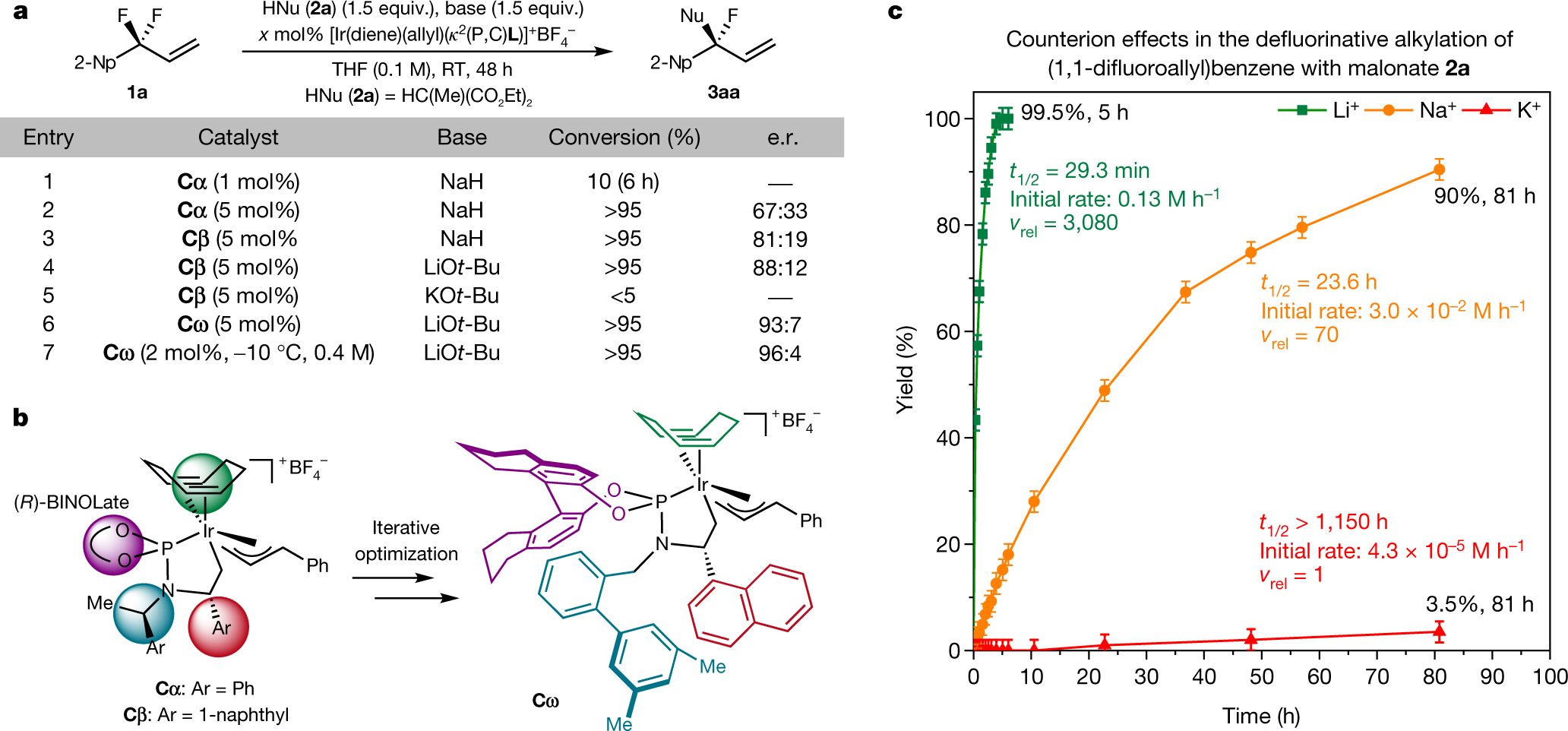
The caption:
It is interesting to note, since this caption mentions chiral HPLC, that fluoropolymers - not that I endorse fluoropolymers in the world as it is - could, conceivably make very hydrophobic chiral stationary phases.
The authors explore the broad synthetic utility of their discovery:
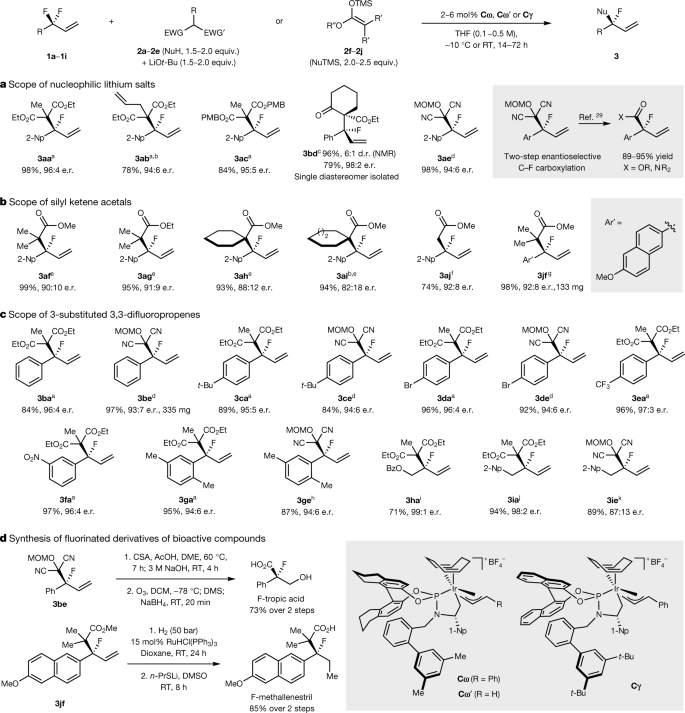
The caption:
The authors extended their work to palladium catalysts:
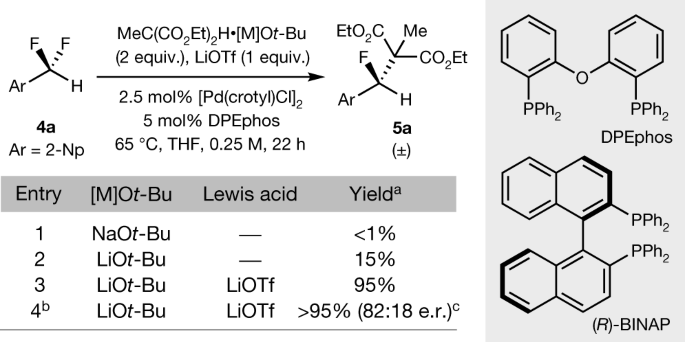
The caption:
Palladium is also a potentially valuable element available from used nuclear fuel, although to minimize its radioactivity - not that radioactive palladium is as much of a problem as people might think, and probably do think in the age of stupidity - it should be isolated very quickly from ruthenium-106 before it is allowed to decay, owing to the long half life of Pd-107.
An exploration of the reaction mechanism.
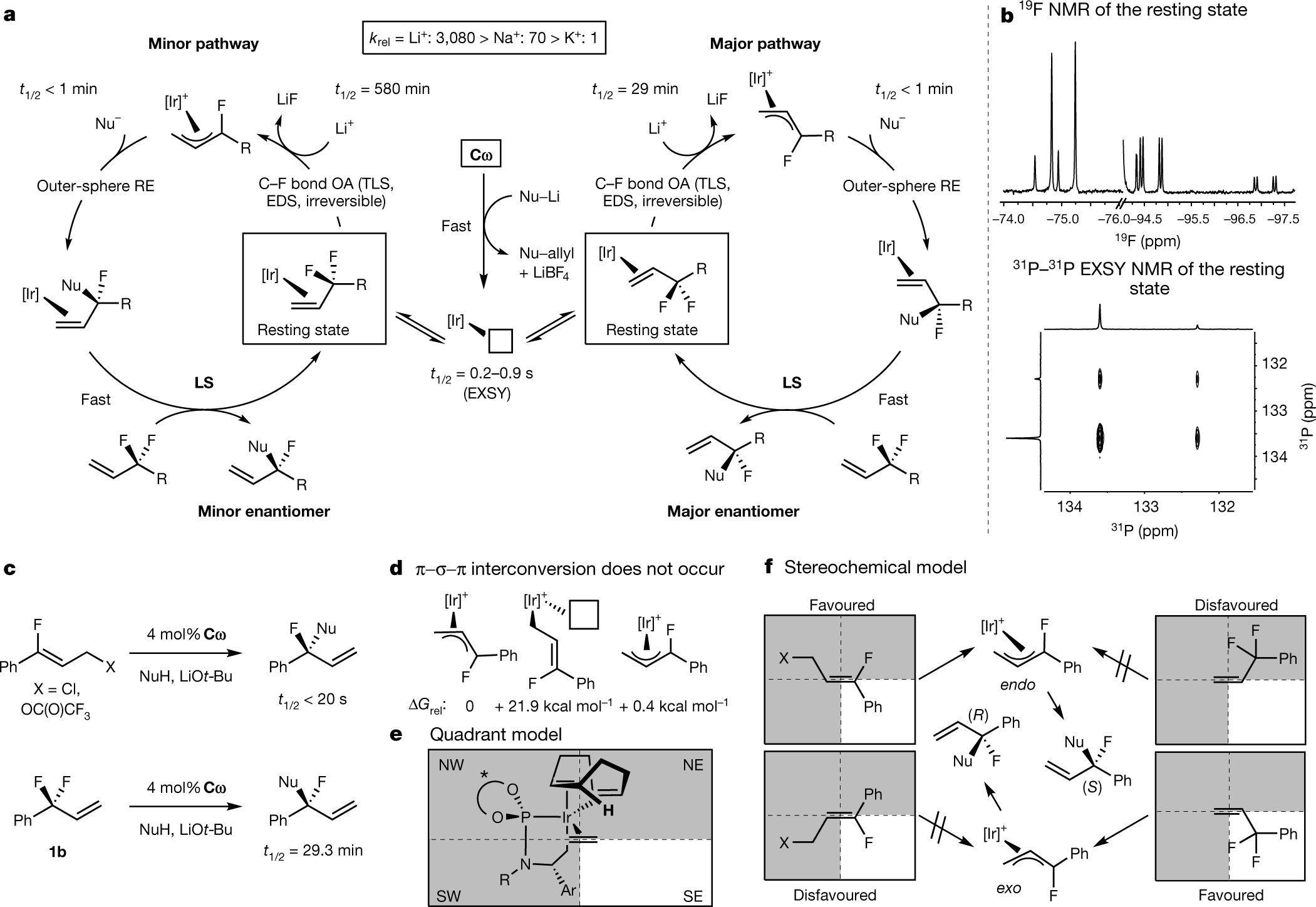
The authors conclude:
This is very beautiful work, rather important I think, and it's a shame that while humanity is at the precipice of a golden age, fear, ignorance and violence are on the rise.
I trust in spite of all the tragedy we are seeing in the winding down of the awful Trump era, despite the pandemic, that you are taking some time to recall how wonderful it is to be alive.
I wish you safety and good health.
Strength through disorder
Obviously the title of this post does not refer to the senile racist orange bastard in the White House who has made America weak through disorder, but instead refers to the subtitle of this paper: Ultrahigh-strength and ductile superlattice alloys with nanoscale disordered interfaces (Yang, Zhao et al., Science 24 Jul 2020: Vol. 369, Issue 6502, pp. 427-432)
I generally avoid thinking about cobalt, since its mining is such a tragedy, although it is possible to imagine synthetic cobalt in some distant future, not one any of us will live to see, but I'm inclined to make an exception here.
The alloy discussed here is a nickel based alloy - as pretty much all superalloys are - but with admixtures of the aforementioned cobalt, iron, aluminum, titanium and boron.
High strength corrosion resistant alloys are essential for the operation of high temperature turbines, which will be a key tool if we are to have even a slight chance of addressing climate change.
I am not sure of all the properties of this alloy, but its main feature is the use of disorder to generate strength, as implied in the subtitle, and perhaps less strictly tied to composition.
From the paper:
Another class of materials that bridge ceramics and metals and which are highly ordered, I feel behooved to mention as they fascinate me and I'm always mentioning them to my son, are the MAX phases, which are generally composed of early period transition metals, carbon or nitrogen, and silicon. But that's a different discussion...
Further on the authors continue...
Figure 1:

The caption:
(A) Bright-field TEM image showing the polycrystalline morphology. (Inset) A corresponding selected-area electron diffraction pattern collected from the grain interior, which shows the L12-type ordered structure. (B) Atomic-resolution HAADF-STEM image and corresponding EDX maps taken from the inner L12-type OSG, revealing the sublattice occupations. (C) High-resolution HAADF-STEM image revealing the ultrathin disordered layer at the grain boundaries with a nanoscale thickness. The images on the right show the corresponding fast Fourier transform (FFT) patterns. (D) EDX maps showing the compositional distribution of the DINL. (E) Schematic illustration highlighting the nanoscale interfacially disordered structure. FCC, face-centered cubic.
DINL = disordered interfacial nanolayer.
Figure 2:

The caption:
(A) Atom maps reconstructed using 3D-APT that show the distribution of each element. Fe, Co, and B are enriched at the DINL, whereas Ni, Al, and Ti are depleted correspondingly. (B) Two-dimensional compositional contour maps revealing the multielement cosegregation behaviors of Fe, Co, and B elements within the DINL. (C) One-dimensional compositional profile that quantitatively reveals the elemental distributions across the OSG and DINL.
OSG = ordered superlattice grain
Figure 3:

The caption:
(A) Tensile stress-strain curve of the NDI-SM tested at 20°C in air. The stress-strain curve of high-strength Ni3Al-type (Ni3Al-2.5 at % B) alloy (9) is also plotted for a direct comparison. (Inset) Tensile fractography showing the ductile dimpled structures. (B) Yield strength (σy) versus uniform elongation (εu) of the present NDI-SM compared with various conventional bulk ordered alloys (8–10, 12, 13, 17, 18, 22–26). (C) Variations of Vickers hardness (HV) of the NDI-SM at elevated temperatures compared with those of conventional ordered alloys (27, 28). (D) Grain size variations as a function of heating durations at a high temperature of 1050°C. (Inset) A typical EBSD inverse pole figure (IPF) map showing the grain size of the sample annealed at 1050°C for 120 hours. The NDI-SMs exhibit an exceptionally high resistance against the thermally driven softening and grain coarsening.
EBSD = electron backscatter diffraction.
This temperature should be sufficient for many applications. Most modern turbines are coated with thermal barrier coatings that allow them to operate at temperatures higher than their melting points.
I like to imagine and dream about systems that operate at temperatures about 1450°C because of my fondness for thermochemical carbon dioxide spitting catalysts, but that's just me.
The conclusion:
The composite architecture of our superlattice alloy, especially the multielement cosegregation-induced interfacial disordering, can be utilized to design high-strength ultrafine-grained or nano-grained materials with enhanced grain-boundary stability and associated coarsening resistance. We anticipate that this approach should be applicable to many other metallic systems, particularly the compositionally complex ordered alloys. This may lead to families of high-temperature structural materials that might avoid some of the drawbacks of high-temperature alloys currently in use. These superlattice materials will be of great interest for a broad range of aerospace, automotive, nuclear power, chemical engineering, and other applications.
A very cool paper out of repressed and dying Hong Kong and increasingly imperial China.
I trust you are doing everything to keep safe and finding space to enjoy life as well.
"Do less medicals, just drop your books and do more gymnastics, dedicate yourself to equestrian...
...sport!"
I was surfing the internet for some historical punctilios, and stumbled upon the execution of Mussolini, whose dead body was famously hung upside down in 1945 in Milan after his execution, with his mistress, by Italian partisans.
Looking at the photograph, I noticed three other bodies, and decided to find out who they were.
One of them was Achille Starace, who was a prominent fascist in the Mussolini regime, and who was responsible for propaganda.
He is recorded as one of the most stupid people in history. From his Wikipedia page:
...On one occasion he had to preside officially at a high-level medical symposium, but, much to the displeasure of the participants, turned up one hour late for the opening address, explaining that he had been on his daily hour of horse riding. Far from offering excuses to the infuriated audience, he sharply cut short the protests with a famous phrase: "do less medicals, just drop your books and do more gymnastics, dedicate yourself to equestrian sports" (datevi all'ippica in Italian). The motto stuck and in the 21st century Datevi all'ippica still is a proverbial catchphrase to tell someone that he is incompetent and should try his hand at some unskilled job.
It seems relevant to discussions in the United States in 2020 somehow.
One One One
?t=77Site-specific glycan analysis of the SARS-CoV-2 spike protein.
The paper I'll discuss in this post is this one: Site-specific glycan analysis of the SARS-CoV-2 spike (Yasunori Watanabe*, Joel D. Allen*, Daniel Wrapp, Jason S. McLellan, Max Crispin, Science, 17 Jul 20, Vol. 369, Issue 6501, pp. 330-333)
In recent years, I've been dragging myself, when I have time, into a consideration of the 4th, and clearly the most challenging, structural motif in molecular biology, following on amino acid based structures (proteins and peptides), nucleic acid derived structures, obviously DNA and RNA, but also including biological energetics, lipids and the complex structural and signalling pathways, especially in the signalling of inflammation, and finally, the difficult one, about which I am working to learn, the sugars. All of these classes of molecules play in the dance of metabolism, the signalling and transformations that make living things be, well, living. In what little spare time I have for it, I have been working to understand glycobiology as well as the structures of the "simple" sugars (which are not necessarily simple), modified sugars, glycosides, ordinary and modifed glycopolymers and their derivatives.
It is worth noting that the largest fraction of the total biomass on this planet is a glycopolymer, cellulose.
Many important molecules in physiology are hybrid molecules containing the core subunits of basic biological motifs. A hybrid molecule containing a sugar bonded in a specific way, via it's oxidized carbon (acetal form) is called a "glycoside." Here is an example of a glycoside that is bonded to a fat, a "GPI anchor":

Lipid Web
A simplified version of this diagram, from the same website, is here:

"GPI anchors" = Glycosylphosphatidylinositol-Anchors
These molecules have three biological motifs represented, four sugars, including an amino sugar, a sugar derived molecule important in physiology, inositol, a diacyl fat, and a protein, which may or may not have other glycosides (glycans) attached. If one looks carefully at the second diagram, where the sugars are designated "Man" (for mannose) and the aminosugar is designated "GlcN" (for glucosamine, one can see that each of the mannoses can be bonded to the other mannose at any of four different positions (since stereochemistry robs mannose of its symmetry) - actually there are five different ways, because one bond would be through an oxygen than can have either of two spacial orientations.
I had the pleasure of working briefly on a GPI anchored protein, alkaline phosphatase, found in the alimentary canal; in the shown example, this GPI anchor works to anchor proteins to the membranes of red blood cells.
Reflection on this point should give an immediate feel for the complexity of systems involving sugars, which should give an appreciation of the sophistication of the paper being discussed.
Glycans on proteins themselves be quite complex; the paper under discussion is about a very complex molecule of this type, a special type of glycoside, " glycan" which is a glycoside of a protein of a specific type that is a key to the understanding of SARS-CoV-2.
Glycans come in two forms, the first and most extensively studied being the N-glycans, which are bonded to proteins at very specific residues, asparagine residues, at the β amide nitrogen. Historically and currently these have been studied by releasing them from the protein using an set enzymes (PGNase) and studying their structure instrumentally in isolation from the parent protein. The other form of glycans, has been somewhat more challenging to release. Nevertheless, the release of glycans for study eliminates the most important information in connection with their function, which is their location on the protein (or peptide chain).
Modern advances in software have gone a long way to address this problem. An example of such software is that of Protein Metrics, the Byonic software. Here is a presentation (on N-Glycans) from that company: Byonic™: N-Linked Glycopeptide Analysis. I recently had the pleasure of watching a scientific webinar by scientists in the groups out which the Science paper comes, the McClellan group and the Crispin group.
There are, of course, other approaches to addressing glycan analysis involving both software and chemistry. I had the pleasure of attending a lecture by Dr. Hui Zhang of Johns Hopkins when she spoke at a conference in New Jersey. Here is an open sourced paper from her group on the subject of O and N glycan analysis: Classification of Tandem Mass Spectra for Identification of N- and O-linked Glycopeptides (Zhang et al., Scientific Reports volume 6, Article number: 37189 (2016))
Anyway, to return to the subject of glycosylation of SARS-Cov-2 S (Spike) Protein.
From the introduction to the paper:
Viral glycosylation has wide-ranging roles in viral pathobiology, including mediating protein folding and stability and shaping viral tropism (9). Glycosylation sites are under selective pressure as they facilitate immune evasion by shielding specific epitopes from antibody neutralization. However, we note the low mutation rate of SARS-CoV-2 and that as yet, there have been no observed mutations to N-linked glycosylation sites (10). Surfaces with an unusually high density of glycans can also enable immune recognition (9, 11, 12). The role of glycosylation in camouflaging immunogenic protein epitopes has been studied for other coronaviruses (10, 13, 14). Coronaviruses form virions by budding into the lumen of endoplasmic reticulum–Golgi intermediate compartments (15, 16). However, observations of complex-type glycans on virally derived material suggests that the viral glycoproteins are subjected to Golgi-resident processing enzymes (13, 17).
The type of analysis performed here, and implied in the discussions above is what we call "bottom up" analysis, which involves the enzymatic digestion (usually trypsin is the enzyme most widely used, although there are others) of a protein into smaller peptide fragments.
Some pictures from the text:

The caption:
(A) Schematic representation of the SARS-CoV-2 S glycoprotein. The positions of N-linked glycosylation sequons (N-X-S/T, where X ≠ P) are shown as branches (N, Asn; X, any residue; S, Ser; T, Thr; P, Pro). Protein domains are illustrated: N-terminal domain (NTD), receptor binding domain (RBD), fusion peptide (FP), heptad repeat 1 (HR1), central helix (CH), connector domain (CD), and transmembrane domain (TM). (B) SDS–polyacrylamide gel electrophoresis analysis of the SARS-CoV-2 S protein (indicated by the arrowhead) expressed in human embryonic kidney (HEK) 293F cells. Lane 1: filtered supernatant from transfected cells; lane 2: flow-through from StrepTactin resin; lane 3: wash from StrepTactin resin; lane 4: elution from StrepTactin resin. (C) Negative-stain EM 2D class averages of the SARS-CoV-2 S protein. 2D class averages of the SARS-CoV-2 S protein are shown, confirming that the protein adopts the trimeric prefusion conformation matching the material used to determine the structure (4).
Mapping of glycans on the SARS-CoV-2 protein:

The schematic illustrates the color code for the principal glycan types that can arise along the maturation pathway from oligomannose- to hybrid- to complex-type glycans. The graphs summarize quantitative mass spectrometric analysis of the glycan population present at individual N-linked glycosylation sites simplified into categories of glycans. The oligomannose-type glycan series (M9 to M5; Man9GlcNAc2 to Man5GlcNAc2) is colored green, afucosylated and fucosylated hybrid-type glycans (hybrid and F hybrid) are dashed pink, and complex glycans are grouped according to the number of antennae and presence of core fucosylation (A1 to FA4) and are colored pink. Unoccupancy of an N-linked glycan site is represented in gray. The pie charts summarize the quantification of these glycans. Glycan sites are colored according to oligomannose-type glycan content, with the glycan sites labeled in green (80 to 100%), orange (30 to 79%), and pink (0 to 29%). An extended version of the site-specific analysis showing the heterogeneity within each category can be found in table S1 and fig. S2. The bar graphs represent the mean quantities of three biological replicates, with error bars representing the standard error of the mean.
Figure 3 invites some commentary after the caption.

The caption:
Representative glycans are modeled onto the prefusion structure of the trimeric SARS-CoV-2 S glycoprotein (PDB ID 6VSB) (4), with one RBD in the “up” conformation and the other two RBDs in the “down” conformation. The glycans are colored according to oligomannose content as defined by the key. ACE2 receptor binding sites are highlighted in light blue. The S1 and S2 subunits are rendered with translucent surface representation, colored light and dark gray, respectively. The flexible loops on which the N74 and N149 glycan sites reside are represented as gray dashed lines, with glycan sites on the loops mapped at their approximate regions.
It is notable that there are many regions in this protein that are not covered by glycans. In virology there is something known as a "glycan shield." We have been working for decades to produce an HIV vaccine. The difficulty in doing that has been informed by the very large glycan shield that covers the HIV virus, which prevents the binding of antibodies to the peptide sequence of the HIV viral proteins. The less extensive glycan shield in SAR-CoV-2, as shown in this cartoon offers hopes for a vaccine.
The authors note as much:
This is a marvelous paper in my opinion, and it shows that we are not technologically disarmed, yet, in the battle against this terrible disease, even if we are temporarily led by an ignorant, obviously emotionally and cognitively impaired anti-science moron.
Our scientific instructure, though damaged, is still intact and there will be time under Joe Biden, to repair it.
I trust you're having a pleasant afternoon despite this being the days of Covid. We are locked inside here in New Jersey because of extreme heat, driven by climate change, an artifact of anti-science on the right, and regrettably on the left as well. I trust you will behave safely, and thus remain safe and well.
Nature's Covid Publications Summary: Covid Antibodies Fade Within Weeks of Recovery.
Nature News is now running a very nice summary of recent Covid research. The caveat to what is contained in it is that most of the research referenced has not been peer reviewed.This week's summary is here: Coronavirus research updates: Antiviral antibodies peter out within weeks after infection (Nature News 7/16/20.
Subtitle: Nature wades through the literature on the new coronavirus — and summarizes key papers as they appear.
It is open sourced.
Some excerpts:
16 July — Antiviral antibodies peter out within weeks after infection
Key antibodies that neutralize the effects of the new coronavirus fall to low levels within months of SARS-CoV-2 infection, according to the most comprehensive study yet.
Neutralizing antibodies can block a pathogen from infecting cells. But such antibody responses against coronaviruses often wane after just a few weeks.
Katie Doores at King’s College London and her colleagues monitored the concentration of neutralizing antibodies against SARS-CoV-2 in 65 infected people for up to 94 days (J. Seow et al. Preprint at medRxiv http://doi.org/d3s2; 2020). In a preprint that has not yet been peer reviewed, the team reports that at the peak of antibody production, people with severe COVID-19 symptoms had higher levels of antibodies than had people with mild disease...
...in most people, antibody levels began to fall about a month after symptoms appeared, sometimes to nearly undetectable levels — raising questions about the durability of vaccines designed to promote the production of neutralizing antibodies...
...15 July — Positive trial results raise hopes for a top vaccine candidate
A leading COVID-19 vaccine candidate generates an immune response against the virus and causes few side effects, according to preliminary data from a phase I safety study with 45 participants...
...The vaccine is being co-developed by Moderna in Cambridge, Massachusetts, and the US National Institute of Allergy and Infectious Diseases. It consists of RNA instructions that prompt human cells to make the virus’s spike protein, generating an immune response.
Lisa Jackson at Kaiser Permanente Washington Health Research Institute in Seattle and her colleagues gave participants two injections, administered four weeks apart, of one of three different doses of the vaccine (L. A. Jackson et al. N. Engl. J. Med. http://doi.org/d3tt; 2020). Most side effects were mild, although three participants who got the highest dose experienced worse complications, such as a high fever...
...5 July — Severe COVID-19 has a telltale immune profile
Scientists have identified an immune-system signature in people with serious COVID-19 — a finding that could inform the development of treatments for the disease.
Benjamin Terrier at the University of Paris and his colleagues analysed blood samples from 50 people infected with SARS-CoV-2 (J. Hadjadj et al. Science http://doi.org/gg4vjx; 2020). Compared to the individuals with mild or moderate symptoms, those with severe disease produced fewer antiviral proteins called interferons and more inflammatory molecules. The researchers also found that blood levels of a specific interferon decreased just before participants had to be taken to intensive-care units...
...and so on...
As one of the world's premier scientific journals, the information probably (but not definitely) has more credibility than what one might read, for example, in the New York Times.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,512