Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Science
Related: About this forumMolten Salt Synthesis of a MAX Phase Derived Porous Carbon for Supercapacitors.
The paper I'll discuss in this post is this one: Molten Salt Electrosynthesis of Cr2AlC-Derived Porous Carbon for Supercapacitor (Zhongya Pang, Xingli Zou, Xiaolu Xiong, Shujuan Wang, Li Ji, Hsien-Yi Hsu, Guangxin Wu, Qian Xu, and Xionggang Lu, ACS Sustainable Chem. Eng. 2019, 7, 12938−12947)
Once, when I was on a job interview a long time ago where I gave a little lecture to the scientific team there, including the CEO, on the interesting statistics of the random sorting beads into containers, the CEO of the company in my private interview with him afterwards, asked me if I thought I could calculate the fraction of the chemical space he was covering with his combinatorial syntheses. I was pretty young and naive then, so I made a stab at an answer, although I'm not convinced that I really had any real practical idea of how to really approach the problem in a meaningful way. I was offered the job, but declined it. Later I met the CEO of another company who had previously worked in that company and told him I declined a job with it whereupon he remarked that I must be a smart man for not taking the job.
Organic chemistry, essentially the chemistry of carbon, represents a vast chemical space, I would guess essentially an infinite space, and in this sense, a discussion of covering a fraction of it is purely absurd, and a discussion of covering it is, in fact, more than a little arrogant. There are people, of course, who manage manage in magnificent minds huge chemical spaces, but I suppose that even the best minds in the world can keep up with only a minuscule portion of what is there.
Staring into that space, the chemical space of possible organic compounds, is a beautiful thing.
Including molecules like the dangerous fossil fuel waste carbon dioxide and other carbon compounds lacking bonds with hydrogen, the inorganic carbon chemistry seems on the surface simpler. When I was in high school, for example, we were taught that there were two allotropes of carbon, diamond and graphite. Actually a third allotrope was all over the place, and despite the vast efforts of thousands of organic chemists to synthesize it with little success, it was discovered in lampblack, a discovery worthy of a Nobel Prize. This is "buckminsterfullerene" usually referred to in these times simply as "fullerene" a C60 approaching spherical symmetry. It turns out that the chemical space of carbon allotropes is vast. There are not just fullerenes, but also a vast array of compounds like graphene, carbon nanotubes, etc. etc.
When one adds other inorganic atoms, not just, oxygen, nitrogen, and sulfur, but pretty much every long lived element in the periodic table, the inorganic chemistry of carbon is also extremely rich.
An extremely interesting area of inorganic chemistry of carbon, although not strictly an allotrope are a class of materials known as MAX phases, the most famous of which is Ti3SiC2, although many other examples exist. The compounds were first synthesized in the middle of the 20th century, but their properties were basically more or less ignored until the Materials Scientist Michel Barsoum of Drexel University rediscovered them and developed them, a major - and in my view although I don't count - and Nobel Prize worthy body of work. The MAX Phases have the interesting property of offering many of the best properties of metals, high strength, fracture resistance and machinability, the ability to conduct electricity, while offering many of the best properties of ceramics, chemical resistance and high temperature resistance,
One of the interesting properties of the MAX phases is that their structure is extremely regular, as is the case in metals, with the special property that the three elements of which they are composed are highly layered. By exploiting the differing chemistry of the elements within, one can make by leaching one (or two) layers out a class of materials called "MAXenes.
The electrical properties of the MAX phases are different than "normal" metals, and a vast amount of work has been done on the topic. For example, if one enters the search terms (MAX phases anisotropy conductivity) into Google Scholar, limiting the search to papers published since 2015, one will get over 17,000 hits.
A capacitor is a device, as many people know, which consists of two conducting plates separated by a non-conducting substance (a dielectric or electrical insulator). The plates in a capacitor are designed to be charged with opposite charges, and in so doing stores electricity. Although I am extremely rusty when it comes to circuit analysis, I do recall that capacitors are widely used in circuits to filter frequencies out of a circuit; this is known as an "RC circuit," which can be utilized to filter out static signals, extraneous resonances, as well as to manage power surges without wasting all of the energy as a zener diode might do. Also, because the charging of a capacitor can be expressed by a separable first order differential equation the solution of which involves an exponential time constant, properly arranged in circuits, capacitors can be utilized to make oscillators, an obviously important feature of circuit design.
As an aside, capacitors can be utilized in certain kinds of detonators, known as exploding bridgewire detonators that can be made to explode in a very precise manner with precise timing. This makes them important components of nuclear weapons, in particular those depending on implosive shockwaves. To my knowledge, however, no one has ever proposed banning capacitors because they can be used in nuclear war.
Capacitors are widely used in many technologies, most famously cell phones, which can contain hundreds of small light weight capacitors that are based on tantalum. Tantalum, like aluminum, generally contains a passive oxide layer on its surface. This oxide is an extreme dielectric, meaning that layering polished tantalum on top of unpolished tantalum sheets produces a very effective capacitor. This is the most common application for the metal. Tantalum is often considered a "conflict metal" since its mining in the Congo region is often under conditions of essential slavery, often involving small children, an ethical consequence of high technology we'd all rather ignore, just as we ignore the identical situation with respect to cobalt while we worship Elon Musk's car for millionaires and billionaires.
The amount of tantalum in a cell phone is rather small, but given that 1 to 2 billion cell phones are produced each year - there are more cell phones than people on this planet - it adds up.
Depending on the type of MAX phase, as layered structures with a metal, for example, titanium, zirconium, niobium, tantalum, etc, either a semimetal such as silicon, gallium, germanium or the metal aluminum, and a nonmetal (carbon or nitrogen), many types of MAX phases are potential capacitors, at least in the case of being coupled reliably with nanoscale leads. A perfectly formed MAX phase such as the famous Ti3SiC2, as it case of a capacitor, is a layered device with alternating conducting and insulating layers.
Here is the structure of Ti3SiC2:
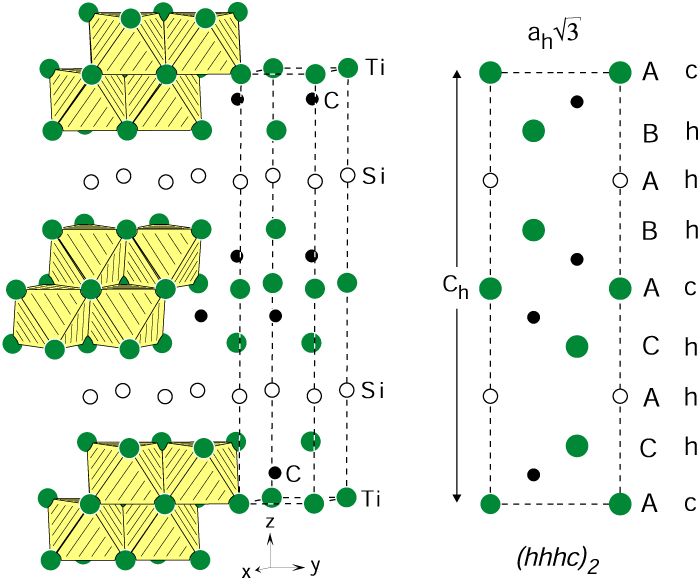
Figure 22: Crystal structure of Ti3SiC2 (space group P63/mmc). The close-packed titanium layers containing the carbon atoms are shown at the left-hand side. The edge-sharing Ti6C octahedra are emphasized. At the right-hand side a (110) cut through the hexagonal cell is shown. The titanium and silicon atoms form close-packed layers with the stacking sequence hhhc, where the silicon atoms correspond to the second h.
Depending on the configuration, in series or in parallel, or a combination thereof, it is possible to make a MAX phase including nanoscale circuitry, into a solid state energy storage device.
Another type of capacitor is the "supercapacitor" which is relevant to the paper mentioned at the outset of this post. Since a capacitor is a device with stable charge separation, it follows that one immersed in a liquid containing ions, or, as in an "ionic liquid" consisting entirely of ions, will, by virtue of charge attraction, also experience charge separation in the fluid. Thus the fluid will, in a sense, be a liquid capacitor as a result, raising the capacitance overall. If the fluid is contained partially within a porous solid phase which is conducting, this solid phase is an electrode, and this charge separation can be utilized to generate a current upon discharge, just like a solid phase capacitor. Under these conditions, one gets "super capacitance," extra bang for the charges in the solid phase.
We generally think of carbon, as a nonmetal as being an insulator. Carbon however has anisotropic conductivity. It conducts when the graphite layers are oriented in one direction, and insulates in the opposite direction. Carbon electrodes are well known and widely utilized, particularly in electrolytic technologies such as that utilized in the aluminum industry, or, in the recently developed FFC Cambridge process which famously can be utilized to produce cheap titanium and many other metals. In a supercapacitor, carbon in various allotropes, nanotubes, graphene, and fullerenes, for example can thus function as an electrode. In order to maximize the containment of ions in the fluid, as well as to allow for large surface areas for discharge, porous carbon is ideal, which is the subject of this paper.
From the introductory text:
The technologies for electrochemical energy storage are now playing an irreplaceable role in both safety and energy reserves.1−5 Supercapacitors as well as Li-ion batteries are the most typical application fields.4−6 Therefore, electrode materials providing structural supports to electrochemical energy-storage devices have been a hot research topic.7−9 Carbon-based materials with excellent chemical and physical properties (stability, nontoxicity, good formability, etc.) have been the most commonly utilized electrode materials for supercapacitors.8,10−12 Generally, the mechanism of supercapacitors with carbon-based electrodes is explained by electrical double-layer theory,8 which can be described as the accumulation and release of electrostatic charge on the interface layer of electrode. Therefore, the performance of the supercapacitors generally relates to the pore structure and surface area of electrodes.8,12−15 Commonly, mesopores ranging from 2 to 50 nm have excellent applicability to different electrolytes and thus can improve the utilization of surface area effectively.15,16 Therefore, obtaining precise control of pores is beneficial for improving the performance of supercapacitors.
Carbide-derived carbon (CDC), prepared by removing metal atoms from carbide precursors, exhibits great potential for the electrode material of supercapacitors because of its excellent properties (such as tunable porosity, structural versatility, and high specific surface area, etc.).17−22 Currently, the primary method utilized for CDC synthesis is chlorination process. Through disposing of the carbide precursors by hot chlorine gas, metal chlorides and derived carbon can be achieved, and the general reaction in this process can be expressed as xMC(s) + (y/2)Cl2(g) → MxCly(g) + xC(s) (where MC is metal carbide, MxCly is gaseous metal chloride).17,23−25 However, this chlorination process inevitably arouses environmental concerns because of chlorine gas. Besides, the pore structure of the CDC produced by the chlorination process generally only consists of narrow micropores, which commonly restrict the electrolyte accesses into the pore structure.19 Actually, effective electrolyte penetration usually comes from the contribution of mesopores, 26 and thus, it is crucial to create mesopores during the etching process. Besides, the chlorination process commonly needs to be operated at high temperature.27 The pore structure can also be influenced by the carbide precursors.21,22 Therefore, seeking a new green process and appropriate precursors for the synthesis of CDC with controllable micropores/mesopores ratio is still necessary...
Carbide-derived carbon (CDC), prepared by removing metal atoms from carbide precursors, exhibits great potential for the electrode material of supercapacitors because of its excellent properties (such as tunable porosity, structural versatility, and high specific surface area, etc.).17−22 Currently, the primary method utilized for CDC synthesis is chlorination process. Through disposing of the carbide precursors by hot chlorine gas, metal chlorides and derived carbon can be achieved, and the general reaction in this process can be expressed as xMC(s) + (y/2)Cl2(g) → MxCly(g) + xC(s) (where MC is metal carbide, MxCly is gaseous metal chloride).17,23−25 However, this chlorination process inevitably arouses environmental concerns because of chlorine gas. Besides, the pore structure of the CDC produced by the chlorination process generally only consists of narrow micropores, which commonly restrict the electrolyte accesses into the pore structure.19 Actually, effective electrolyte penetration usually comes from the contribution of mesopores, 26 and thus, it is crucial to create mesopores during the etching process. Besides, the chlorination process commonly needs to be operated at high temperature.27 The pore structure can also be influenced by the carbide precursors.21,22 Therefore, seeking a new green process and appropriate precursors for the synthesis of CDC with controllable micropores/mesopores ratio is still necessary...
What follows is a description of the MAX phase Cr2AlC, a max phase with two metals and a (nominal) nonconductor, as opposed to Ti2SiC3 which has two insulators and one metal.
The structure of these materials will be shown in some of the graphics shown below. What is interesting in this process is that it is a molten salt etching process.
Molten salts are just what they sound like, salts heated to beyond their melting points. The technology of using these is not particularly exotic; they have been industrial use for quite some time, in the Hall process, to which I alluded above for the production of aluminum, and the also mentioned FFC Cambridge process. The production of fluorine gas has long utilized molten salts, a mixture of KF and HF in a ratio of 1 : 2.30 in an electrolytic process; it also works with rubidium and cesium albeit at higher cost. A rather famous nuclear reactor operated in the 1960's, the molten salt reactor (MSR) which utilized a low melting eutectic of BF3 and LiF known as FLIBE. (I used to be very fond of these types of reactors, but I don't think now that they are the best kind of reactors possible; I would hate to be in a position of advocating for the expansion of mining beryllium, a notoriously toxic metal.) The discovery of a wide array of what have come to be known as "ionic liquids" - some exist naturally in living systems, for example choline choride) has vastly expanded, essentially to infinity, the number and types of molten salts that can exist.
In this paper a "traditional" molten salt has been used, one very similar to that used in the FFC Cambridge process.
From the experimental text:
Cr2AlC (∼25 μm) powder supplied by Forsman Scientific (Beijing) Co. Ltd. was well-mixed with 20 wt % polyvinyl butyral binder through ball milling for about 12 h. The mixture powders were compressed under 30 MPa to prepare cylindrical pellets (10 mm in diameter), each weighing 0.5 g. Then, a graphite rod (15 mm in diameter) and the Cr2AlC pellet sandwiched between two graphite plates were fastened to Fe−Cr−Al wires, which were employed as the cathode and anode, respectively. The assembled electrodes were placed in a corundum crucible to form the electrolytic cell. A molar ratio of 1:1 of anhydrous CaCl2:NaCl eutectic mixture was used as electrolyte. Whole experiments were carried out in high-purity argon atmosphere. To purify the molten CaCl2/NaCl, a pre-electrolysis experiment was performed (2.8 V, 3 h). The electrochemical etching process was conducted separately at 3.0 V and different electrolytic temperatures. After the etching process, the samples that were cooled to room temperature were disposed with distilled water and then dried at low temperature.
Some graphics and pictures from the text:

The caption:
Figure 1. (a) Schematic illustration of the electrolytic apparatus and the structures of Cr2AlC and the theoretical Cr2AlC-CDC after the etching process. (b) XRD patterns of Cr2AlC and the Cr2AlC-CDC obtained at different temperatures. (c) The (002) interplanar spacing of the CDC samples calculated according to Bragg’s formula; the inset is the molecular structure of the graphite.

The caption:
Figure 2. (a) Raman spectra and (b) full widths at half-maximum of G peak, intensity ratio of D peak and G peak (ID/IG) of the CDC obtained at different temperatures. (c) N2 adsorption/desorption isotherms and (d) pore size distribution of the CDC synthesized at different temperatures.

The caption:
Figure 3. (a) SEM image of Cr2AlC precursor. SEM images (b−e) and HR-TEM images (f−i) of the CDC obtained under different temperatures: 600 °C (b,f), 700 °C (c,g), 800 °C (d,h), and 900 °C (e,i).

The caption:
Figure 4. Schematic for (a) Cr2AlC, (b) Cr2C, and (c) AlC (orange represents Al atom, gray represents C atom, and purple represents Cr atom).

The caption:
Figure 5. (a) Clean surface for Cr2AlC (100) surface. Atomic oxygen adsorption on (b) Cr−Cr bridge and (c) Al−Al bridge

The caption:
Figure 6. (a) Current−time curve of the synthesis of CDC at 3.0 V and 600 °C; the insets are the photos of Cr2AlC powder (i), Cr2AlC pellet (ii), and CDC product (iii). (b) XRD patterns of the partially etched Cr2AlC at 3.0 V and different parameters, (i) 900 °C, 3 h, (ii) 900 °C, 0.5 h, (iii) 600 °C, 3 h, (iv) 600 °C, 0.5 h. (c) SEM image of the cross-section of the partially etched Cr2AlC at 600 °C and its corresponding elemental maps (d−g). SEM images of the sample obtained at 3.0 V and 600 °C for 0.5 h (h) and 3 h (i), and their corresponding EDX spectra detected over the marked points (j−m). Elemental Pt comes from the spray-platinum (Pt) treatment process.

The caption:
Figure 7. (a) Schematic diagram of the transformation of the anode pellets through electrochemical etching. (b) Schematic illustration of the formation process of Cr2AlC-CDC in molten salt during the etching process.

Figure 8. Electrochemical performance of the Cr2AlC-CDC obtained at 600 °C. (a) Cyclic voltammetry curves at different scan rates. (b) Galvanostatic charge/discharge curves at different current densities; (c) Cycling performance at 500 mA g−1 and (d) the specific capacity at different current densities. The electrochemical performance of the Cr2AlC-CDC obtained at different temperatures. (e) Cyclic voltammetry curves tested at a scan rate of 10 mV s−1. (d) Nyquist plots measured between 10 mHz and 100 kHz.
From the conclusion, summing up what went on in the full paper:
In conclusion, porous CDC has been synthesized by electrochemical etching of Cr2AlC in molten CaCl2/NaCl. We have systematically investigated the characteristics of the products obtained at different experimental parameters. The results show that the CDC obtained at 600 °C is mainly amorphous carbon with rich micro/mesopores and the largest specific surface area (1343 m2 g−1). High temperature can facilitate the conversion from disordered carbon into graphitic carbon. In addition, the specific surface area decreases with increasing temperature, whereas the pore size increases with increasing temperature, which can be utilized to adjust the pore structure. DFT analysis shows that Cr2C structure is relatively stable with the formation energy of −0.57 eV/atom compared with AlC structure of −9.50 eV/atom. DFT calculation and intermediate products analysis indicate that the synthesis pathway of CDC includes the electro-oxidation process and electrochemical etching process. In addition, the as-synthesized CDC exhibits outstanding electrochemical performance for supercapacitors, and the specific capacitance of the CDC obtained at 600 °C can reach 183 F g−1 at 500 mA g−1 and remains at 98.2% after 5000 cycles, demonstrating excellent stability.
Super capacitors have been the subject of much attention in the popular "wishful thinking" memes that go around endlessly as the atmosphere collapses relating to so called "renewable energy," a trivial and useless form of energy that has not addressed climate change, is not addressing climate change and will not address climate change. All of this attention has been directed toward bourgeois fantasies about storing energy from wind and solar energy which is notoriously unreliable without it (and even more environmentally questionable with it).
Energy storage, whether in a battery or in a capacitor, wastes energy. Capacitors are devices which generate significant heat, one can see pressure release expansion disks on some which are designed to prevent them from exploding, except of course, as mentioned above, in precisely timed detonators. It is one thing to have capacitors in circuits designed to accomplish some task, as in the operation of communication devices and computers, another to storing so called renewable energy. There has never, not once, been significant so called "renewable energy" to store in any case; the combined wind, solar, geothermal and tidal energy output has yet to produce 2% of the nearly 600 exajoules of energy humanity consumes each year. The use of dangerous fossil fuels is accelerating, not coming to an end. Dangerous natural gas is not "transitional;" it is increasing ensconced as an unconscionable burden on all future generations. Representations to the contrary are simply pretty lies we tell ourselves.
This realization is beginning to emerge from the shadows, even among those not enthralled by the international rise of simplistic fascist and racist impulses which while not helping the situation, are hardly uniquely responsible for it.
In any case, this little somewhat meaningless scientific aside is, if nothing else, interesting.
I trust you're enjoying your workweek.
InfoView thread info, including edit history
TrashPut this thread in your Trash Can (My DU » Trash Can)
BookmarkAdd this thread to your Bookmarks (My DU » Bookmarks)
0 replies, 1209 views
ShareGet links to this post and/or share on social media
AlertAlert this post for a rule violation
PowersThere are no powers you can use on this post
EditCannot edit other people's posts
ReplyReply to this post
EditCannot edit other people's posts
Rec (5)
ReplyReply to this post