Science
Related: About this forumCritical Masses of the Three Accessible Americium Isotopes.
Last edited Sat May 15, 2021, 05:38 AM - Edit history (1)
Despite the fact that the overwhelming number of war deaths in the last century have resulted from the diversion of petroleum products to make weapons of mass destruction, and zero deaths have occurred from the diversion of commercial nuclear fuel to make weapons of mass destruction, a great deal of attention has been paid to the concept of "critical mass" of actinides, as if it were simple to make nuclear weapons.
Anti-nuke rhetoric, which has made it technically unfeasible to address the far more serious and far more likely disaster scenario of mass destruction by climate change, albeit less instantaneous, as opposed to wholesale nuclear war, often includes silly and frankly absurd calculations about "how many" nuclear weapons can be built from used nuclear fuel.
The reality is that no one can make a nuclear weapon in one's kitchen, and without sophisticated and expensive equipment, anyone handling actinides other than uranium, a naturally occurring product, would face extreme danger to their person without taking extensive precautions.
This is why terrorists like the right wing zealot Timothy McVeigh, and the Saudi Arabian 9/11 terrorists utilized petroleum based weapons of mass destruction, diesel fuel and (dangerous natural gas generated) ammonium nitrate in McVeigh's case, and jet fuel in the Saudi terrorist's case.
As I pointed out elsewhere, it will never be possible to make nuclear war impossible, since uranium exists, and it will never be possible to consume all of the uranium on Earth: On Plutonium, Nuclear War, and Nuclear Peace
The number of extant critical masses that people who are, frankly, complete idiots, utilize in their appeals to fear and ignorance with respect to nuclear wars to advocate against "going nuclear" to address climate change - even though it is the only feasible means of doing so - has an alternate meaning however. The number of critical masses obtainable also represents the number of small nuclear reactors that can be built in a "breed and burn" sense which would render all of the world's depleted uranium and all of the world's waste thorium into valuable fuels with the potential to shut all energy related mining facilities indefinitely.
I favor the uranium/plutonium nuclear fuel cycle on the grounds that it is infinitely sustainable. I don't have anything against the thorium/U-233 cycle, but I think that because of geochemical quirks, it is possible to imagine the depletion of recoverable thorium resources because of thorium's low solubility in seawater when compared to uranium.
For many years, a few decades actually, I considered that there was not enough plutonium to immediately displace all the world's dangerous coal, dangerous petroleum, and dangerous natural gas. When I became aware of the "breed and burn" concept however, I realized that "enough" is simply a function of the number of available critical masses, meaning that the elimination of the use of dangerous fossil fuels is possible almost immediately, in the absence of stupidity, although the absence of stupidity may itself be inaccessible.
There is "enough," particularly in the desirable case of nuclear weapons disarmament.
Because used nuclear fuels have foolishly not been reprocessed over the more than half a century of accumulation in an atmosphere of fear and ignorance, much of the extremely valuable plutonium-241 formed in them, has been allowed to decay to Americium-241. Americium-241 is often thought to be a "difficult" nuclide, because it decays, producing a significant heat load in the process to neptunium-237, which in turn, is theoretically mobile in putative waste dumps, although the idea of having waste dumps for valuable nuclear fuel should be regarded as absurd, since all of the components of used nuclear fuel represent valuable resources.
Except for uranium-238 and thorium-232, both of which occur naturally in vast quantities, and some very rare isotopes of actinium, present in trace amounts in uranium ores, all of the actinides subject to isolation in macroscopic amounts have a critical mass in a fast (unmoderated) neutron spectrum.
As a nuclear fuel, Americium lacks some of the attractive features (to me anyway) present in plutonium and neptunium as nuclear fuels. Specifically the melting point of Americium is higher than either of these two actinide elements. (A plutonium/neptunium eutectic has the lowest melting point among the actinide elements subject to isolation.)
Nevertheless, as a source of denaturing isotopes of plutonium, plutonium-238 and plutonium-240 and plutonium-242, the use of americium fuels has much to recommend it.
In 2003, at a conference on nuclear fuels, British authors, Henmanth Dias, Nigel Tancock, and Angela Clayton, offered a paper that recalculated the critical masses of Americium isotopes.
Three such isotopes will appear in Americium isolated from any source, Am-241, Am-242m, and Am-243. It is exceedingly difficult to separate the isotopes of americium, since it does not form volatile compounds. The shortest lived isotope just listed is Am-242m, which has a half-life, off the top of my head, of around 152 years. It is the least available of all Americium isotopes, since the majority of the 242 isotope formed in nuclear reactors is Am-242, the nuclear isomer of Am-242m, which has a half-life, again off the top of my head, of 16 hours. The 242 isotopes, both of them, are the most fissionable of all Americium isotopes.
Here are the calculated critical masses of Americium isotopes using various nuclear codes:
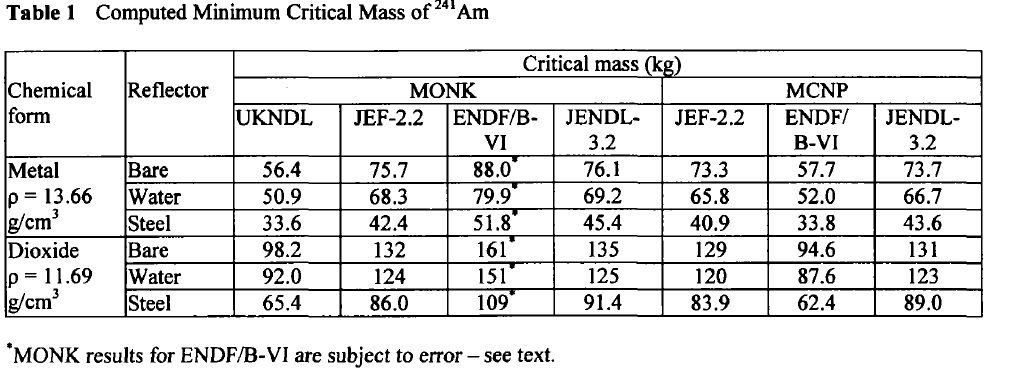
Am-241:
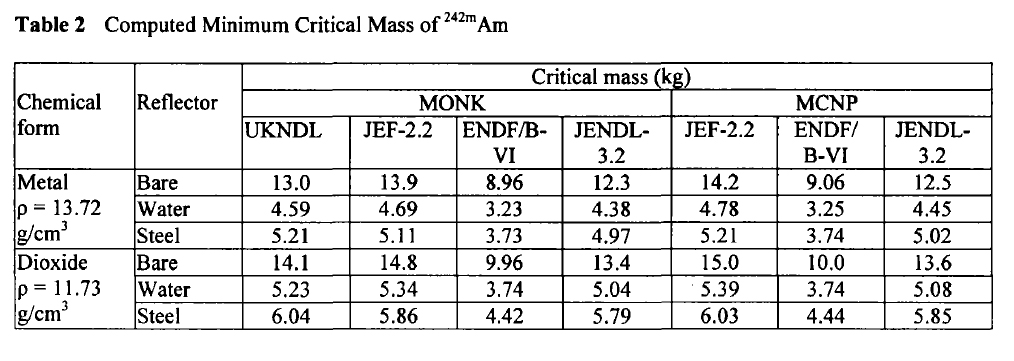
Am-242m:
Am-243:
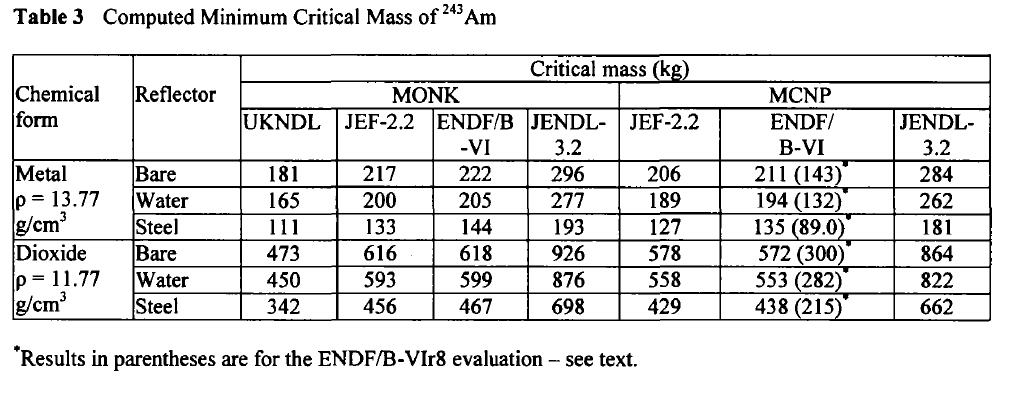
These tables are from the Proceedings of the JAERI conference 2003, pages 618-623.
I hope your weekend is wonderful; and that your plans for the upcoming Thanksgiving holiday are proceeding satisfactorily.
SCantiGOP
(13,870 posts)Americium isotopes just the other day
![]()
NNadir
(33,518 posts)I personally usually think about Americium several times a day, every day, but my favorite element to think about is plutonium, since it's just so fascinating.
Other elements I think about regularly are of course, carbon, and titanium and cesium, neptunium, cerium, cobalt, strontium, barium, and fluorine.
My son used to joke with me when we were touring universities and we'd walk down the halls of academic buildings to view the posters and there'd be some poster about say, zirconium, and I'd say, "that's my favorite element," and he'd say, "Dad, every element is your favorite element..."
It's kind of true.
Don't worry about your smoke detector going critical. It won't.
I_UndergroundPanther
(12,470 posts)Thanks for the info.
Fascinating.