NNadir
NNadir's JournalBil je moski, vzemite ga za vse: Stefan Flow in Supercritical Water.
Bil je moški, vzemite ga za vse. Ne bom več gledal nanj podobnega.
I came across this paper yesterday: Direct Numerical Simulation of Fluid Flow and Heat Transfer of a Reactive Particle Layer with Stefan Flow in Supercritical Water Yingdong Wang, Huibo Wang, and Hui Jin Industrial & Engineering Chemistry Research 2023 62 (3), 1636-1645.
As the properties of supercritical water always catches my eye, I realized that I had no idea what "Stefan flow" is. (It turns out it's something I probably should have known, but didn't.) It is a flow in which a solid material is taken up in a fluid in motion by an action at the fluid/solid interface. Although the word is not exactly equivalent, abrasion touches somewhat on the topic.
Introductory courses in Quantum Mechanics generally introduce early on the Stefan-Boltzmann law which describes the power of emitted radiation by a "black body" at a given temperature, which is proportional to the 4th power of the absolute temperature.
Recalling this from my youth, it occurred to me that I never actually knew anything about who Stefan might be, Boltzmann yes, Stefan no.
Ludwig Boltzmann, who committed suicide in 1906, is one of the most important scientists ever to have lived in my view. He developed the formal understanding of entropy, and in many ways was the founder of statistical mechanics. A consideration of entropy is the key to understanding of energy technology, and energy technology is the most important issue, at least in my view, before humanity today. Nothing else will matter much if we do not address this issue and address it fast. (It's not looking good folks, but we must do what we can.)
It turns out that Boltzmann studied, for the equivalent of a Ph.D. under Joseph Stefan, building on Stefan's work in Thermodynamics.
Who was Stefan?
Interesting guy. A poet, a mathematician, and a physicist in the old Austrian-Hungarian Empire.
His native language was Slovene.
Thus the quotation from Shakespeare translated into Slovene: "He was a man, all in all. I shall not see his like again."
I certainly didn't know that one of the greatest scientists ever to have lived was trained by a Slovenian.
This is a trivial post, but it struck me somehow as something worth saying, that every culture enriches us, whether we know it or not.
I swear I didn't write this article, but offered the chance, I might very well have done so.
The hyper-emotional narrative and negativity around nuclear energy is not accidentalThe intro sounds like a paraphrase of many of my posts:
As German foreign minister Annalena Baerbock noted last week, ‘We buy 50% of our coal from Russia. If we exclude Russia from SWIFT the lights in Germany will go out’.
This caused outrage on social media, but for the wrong reasons. Losing access to electricity is a big deal: blackouts are serious and harm human health. The poor decision-making which has made these countries dependent on a dictator is another issue – and one that should incense us all.
What went wrong? Germany and Italy chose to phase out their nuclear power stations, opting to become reliant on Russian imports instead.
Fossil fuels are not safe or clean – they are causing the destruction of our planet and doing immense harm to our wellbeing. It seems obvious, but while people nod along when you say it, the minute you suggest that, just maybe, nuclear is a better alternative, the conversation changes...
I'm scrambling the below quotes out of order, but they all hit high notes about reality.
And I certainly have noted Gerhardt Schroeder leaving the German Chancellor role to work for Putin:
The earlier remark in the article are new to me, but I certainly consider them accurate:
As I noted recently of Germany, it's now entered the Orwellian "Green is black" realm.
I didn't know that Greenpeace was in the gas business, but lacking any respect for any organization who offers the rote claim that these people give a shit about the environment, that they are "environmentalists." They are, in fact, a cause of climate change, and the way they raise money is tantamount to the Republican Party raising money to fight racism and sexism:
At least, in a rare statement of truth they state clearly that they have no problem with gas, in fact, they're willing to make money off it.
I do, I have a big, big, big problem with gas, but these fuckers don't.
But proof I did not write this article can be found here:
I would have never written the statement I placed in bold.
I don't think we "need" renewables. They're useless, and the word "renewable" applied to solar and wind energy is a pure oxymoron. They not only entrench dangerous fossil fuels, they serve mining interests and they are not sustainable in any form because of the onerous mass and land requirements. Energy dependence on weather failed to provide for humanity back in the early 19th century; it was the "fix" that caused the problem, fossil fuels.
Now we have ersatz "environmentalists," given how our "...but her emails..." media refers to Greenpeace, who have no problem with fossil fuels, even though they are killing the planet (and people), actively, and at doing so an accelerating rate.
All this this said, I agree with the first sentence in the paragraph of the last excerpt, and make this statement all the time. The rise of the antivax movement has drawn the mentality of the antinuke set into full relief in my opinion.
We've been had.
Thermal Mineralization of the Forever Pollutant Perfluorooctanyl Sulfonate to CO2, HF, and SO2.
The paper I'll briefly discuss in this post is this one: Thermal Mineralization of Perfluorooctanesulfonic Acid (PFOS) to HF, CO2, and SO2 Nathan H. Weber, Cameron S. Delva, Sebastian P. Stockenhuber, Charles C. Grimison, John A. Lucas, John C. Mackie, Michael Stockenhuber, and Eric M. Kennedy Industrial & Engineering Chemistry Research 2023 62 (2), 881-892.
It's probably not getting as much attention as the other major threats to the environment diffuse threats to the environment, specifically climate change, about which humanity is doing essentially nothing at all other than jawboning about so called "renewable energy," a failure, or as much attention of a slightly more difficult problem, micro and nano plastics in bodies of water, aerosols, and land, but the problem of perfluoro compounds is increasingly dire.
The introduction to the paper states the problem fairly well:
Thermal decomposition is one of the leading treatment methods, and this process generally comprises operation of two distinct and critical elevated temperature stages. (14) The first stage adopted to thermally remediate PFAS from a solid (e.g., soil) typically uses a rotary dryer operating between 200 and 700 °C, leading to the desorption of PFAS into the gas phase. The now-exiting concentrated PFAS gas stream enters the second stage, which is generally a high-temperature reactor (above 900 °C) used to thermally decompose the PFAS into hydrogen fluoride (HF), carbon dioxide (CO2), and sulfur dioxide (SO2). The second stage has been studied by the USEPA, using the so-called combustor “Rainbow furnace,” where both CHF3 and C2F6 had a destruction efficiency of over 99%. (15) Most thermal treatment methods aim to mineralize all the PFAS into HF, CO2 and SO2 and avoid undesirable fluorocarbons products. Additionally, with thermal treatment plants (thermal desorption plants) being built or currently operating, it is critical that the thermal decomposition of PFOS is well-understood. (16)
Under inert conditions, PFOS was initially found to decompose into HF, SO2, and perfluorooctanyl fluoride (C8F16O). (17−19) However, our most recent inert gas pyrolysis study (20) found that tetrafluoroethylene (C2F4) was also produced from the thermal decomposition of PFOS. This led to the discovery of a previously undetected fission route for PFOS into C8F17 and HOSO2 radicals. The C8F17 radical was found to fission into CF2 radicals and directly into C2F4. Additionally, C8F16O was also found to fission into FCO and C7F15, which will also rapidly fission into CF2 radicals, which rapidly form C2F4 in inert conditions. For the HOSO2 radical, it was found to decompose and form OH and SO2. (20)...
The authors set out to study the decomposition of PFOS in air.
This study is particularly of interest to me, since I have been considering the question of whether Brayton cycle nuclear power plants with air a working fluid, might function as "industrial livers" to detoxify the atmosphere, in effect work as "anti-pollution" machines. From a technical engineering viewpoint - one which I will not live to see, but perhaps my son will - the major problems with this idea, which will have the additive advantage of more than doubling (in a process intensification scheme) the thermal efficiency of nuclear plants, making electricity a side product of plant operations - involves materials science. A combinatorial optimization of heat resistance, corrosion resistance (chemical inertness), and heat transfer (thermal conductivity) is required for materials. I personally believe viable solutions exist considering recent developments of which my son has made me aware.
There is considerable technical information in the paper about chemical, thermodynamic and quantum mechanical modeling, but I will not focus on these but merely focus on some graphics from the paper, with a little commentary.
The first indicates the detection of the decomposition products, including some intermediates not discussed in the title:

The caption:
Figure 1. FT-IR spectra of PFOS thermal decomposition in an air bath gas between 500 and 1000 °C at a 150 mL min–1 (1.5–0.85 s) in a α-alumina reactor.
Note that two of the products shown are highly toxic, HF and COF2 (fluorophosgene). As it happens, both of these products are already found in the planetary atmosphere as currently - although not proceeding at a rate of acceptable speed to prevent accumulation - the main sink for fluorinated species, including HFC's (refrigerants and greenhouse gases), residual CFC's (banned under the Montreal Protocol), and airborne PFAS, is radiation, specifically UV radiation in the upper atmosphere.
I worked in my days on the bench, before becoming an oblivious jet setting executive, with the chloroanalogue of fluorophosgene, generally simply called "phosgene" without reference to chlorine. Typically it is easily decomposed by exposure to base; ammonia will do. I utilized both ammonia and sodium hydroxide, decomposing phosgene into ammonium chloride, ammonium carbonate, sodium carbonate and sodium chloride. The same approach works for fluorophosgene, which is why in my thought experiments on an industrial liver, I include saturated solutions of barium hydroxide (obtainable as a fission product and also from barite minerals) in the line. Barium fluoride is completely insoluble in water, and in fact, does occur naturally as the rare mineral Frankdicksonite. (Fluorine is mined generally in the form of the calcium analogue of Frankdicksonite, fluorite, CaF2, which is also insoluble.
Although the environmental and health risks of fluorinated species are considerable, particularly as they are subject to physiological concentration, their environmental concentrations, often at the level of parts per trillion, the concentration of the metastable decomposition products, fluorophosgene and HF are unlikely to represent the highest risks. HF is neutralized by various calcium minerals and salts. In the lab we frequently used calcium carbonate, calcium oxide, or in cases of inadvertent dermal exposure, calcium gluconate to neutralize it. In the atmosphere, fluorophosgene reacts with water vapor to generate HF and carbon dioxide.
This technologies are readily available in a putative "industrial liver."
As for SO2, it is a serious pollutant involved with the combustion of dangerous coal, and sometimes dangerous natural gas. It is generated in vastly larger quantities than will apply here in places like, say Germany, where the government has decided to kill people by shutting their nuclear plants and replacing them with coal.
Some other graphics:

The caption:

The caption:

The caption:
"DRE" stands for "Destruction and Removal Efficiency" said to approach 99.9% at high enough temperatures.

The caption:
I find this paper inspiring. I would like to note that there are other options for the destructions of the general class of PFAS, (here an abbreviation for perfluoroalkyl substances). In other matrices, I have focused on radiation from fission products as a tool for ameliorating this serious environmental artifact of "civilization," as the tool for their destruction. I've written a number of posts in this space on that topic.
I trust you're having a pleasant Sunday.
Having Hired 1200 Last Year, the Czech's Energy Utility Make Plans for 2600 Nuclear Professionals.
CEZ recruits for its new build plansIt's a rather short article, the introductory paragraphs:
Last year the company hired 1212 workers, it said, with one in six working in a nuclear team. That means they support either the ongoing generation of electricity at the Temelin and Dukovany nuclear power plants or have joined the teams developing plans for a new large reactor at Dukovany or small reactors at Temelin.
CEZ is currently evaluating bids from Westinghouse, EDF and Korea Hydro & Nuclear Power for the construction of a new reactor at Dukovany. Near Temelin, an area has been designated the South Bohemia Nuclear Park and earmarked for small reactors to operate in the early 2030s...
Improved Dissolution of Plutonium Bearing Fuels Using Biodegradable Solvents.
The paper I'll briefly discuss in this post is this one: Biodegradable Methane Sulfonic Acid-Based Nonaqueous Dissolution, Estimation, and Recovery: Toward Development of a Simplified Scheme for Plutonium-Bearing Fuel Matrices Shiny S. Kumar, Ashutosh Srivastava, and Ankita Rao Industrial & Engineering Chemistry Research 2023 62 (1), 660-669
Plutonium dioxide, like the compound often utilized to model it, cerium dioxide, is an extremely insoluble compound, owing to its tendency to form polymeric species. During reprocessing, which up to this time has involved the PUREX process - a process I personally think is probably outdated as better reprocessing chemistries clearly exist - the formation of plutonium oxide polymers can be and often is problematic.
The Purex process involves nitric acid dissolution of chopped fuel rods, and then partition of fission products and actinides via solvent extraction, generally into kerosene using complexing agents, historically tributyl phosphate. Many other complexing agents have been studied and or utilized, and a variety of modifications of the Purex process have been developed with a number of names such as UREX, Talspeak, Diamex, Ganex, and Truex. The particular details do not matter; they are all largely solvent extraction procedures.
Whether I, and many others believe, that solvent extraction should go away, the reality is that the recovery of plutonium is an essential factor in saving the world and like it or not, most of it has accumulated in used nuclear fuels the overwhelming majority of which are oxide fuels. It is thus with interest that I came across the cited paper which to my knowledge is quite novel. I'm not going to spend a tremendous time discussing it, because I don't have the time and it's in any case, very esoteric, but I thought that the opening paragraphs about the Indian nuclear program, which is in my view, quite innovative in the sense that it involves the best thermal reactor there is, the Pressurized Heavy Water Reactor, the only thermal reactor currently available that is a breeder, albeit using thorium.
The opening paragraphs:
U and Pu assay methods based on neutron activation analysis, X-ray fluorescence, spectrophotometry, radiometry, and mass spectrometry are used at different stages of fuel fabrication and reprocessing. (4−7) However, stringent specifications for the fuel materials at all fabrication stages are ensured by chemical quality control (CQC). (8) Electrometric methods are preferred for bulk assay of U and Pu as a part of the CQC exercise owing to high accuracy and precision offered as well as fast throughput and simple instrumentation requirement. (8) The first step of this method involves matrix-dependent dissolution process employing mineral acids (hydrofluoric acid–nitric acid or sulfuric acid–nitric acid) along with heat treatment [infrared (IR) lamp/reflux]. This is followed by the second step of redox titrimetric determination of U and Pu which mandates addition of different reagents. U and Pu are determined separately by Ti(III) reduction method and AgO oxidation method, respectively. (8−11) Large volume of analytical solution is thus generated which comprises of U and Pu along with several other metal ions (Ti, Ag, Fe, Cr, etc.) in mineral acid medium and requires multiple cumbersome steps for the recovery and purification of Pu. Several “green” approaches for dissolution and recovery of actinide matrices based on supercritical carbon dioxide extraction, room-temperature ionic liquids (RTILs), and deep eutectic solvents (DESs) have been reported. (12−18) Separate studies on reagent-free voltammetric determination of U and Pu on modified electrodes have been carried out. (19−21) However, there is no report addressing the challenge of simplification of the entire scheme, comprising dissolution, determination, and recovery, for the bulk assay of Pu-bearing fuel matrices.
The aim of the present study is to explore a universal, matrix-independent, and non-aqueous method for dissolution and reagent-free determination followed by the simplified route for recovery of Pu. Methane sulfonic acid (MSA) is a biodegradable organic acid that is part of the natural “sulfur cycle.” It is, therefore, considered as a green solvent. (22) It is a well-known solvent for the electroplating industry. However, neat MSA has been explored for the first time in the present study for the dissolution of plutonium-bearing solid matrices. Hence, conditions were optimized for quantitative dissolution of the solid matrices, viz., PuO2, (U,Pu)O2, and (U,Pu)C in MSA, with simple IR heating. Redox speciation of Pu and U in MSA, being a novel system, was studied by UV–visible spectrophotometry and electrometry. Attempts were made to understand the mechanism of MSA–U complexation by IR spectroscopy and water content determination. Subsequently, feasibility was established for reagent-free differential pulse voltammetry-based method for simultaneous determination of U and Pu on a commercially available glassy carbon (GC) electrode. After determination, Pu can be precipitated from MSA medium as oxalate with a Pu removal of >98%. It is noteworthy that the present study also unlocks the prospect of application of MSA in the back-end of the fuel cycle for spent nuclear fuel dissolution and selective actinide separation owing to its strong dissolution power and stabilization of specific oxidation state of metal ions.
India's fast breeder reactor will utilize carbide fuels; the proposed process is flexible enough to incorporate these.
(I'm a nitride and metallic fuel kind of guy, more metallic than nitride, since I think we should exploit modern materials science to exploit some of plutonium metal's less appreciated remarkable properties, but I don't really have a big problem with carbides with the caveat that they tend to moderate neutrons, something less than desirable for breeding purposes, as it reduces neutron economy.)
The authors have a nice table of data from their experiments:

The authors also discuss a lot of wonderful analytical chemistry; regrettably I won't have time to go into it.
It is a lab scale process for now, but probably worth consideration for scale up and piloting. A photograph of some dissolved plutonium:

The caption:
A sort of stripped down process diagram concludes the paper, showing as many process flow diagrams do not the PAT, process analytical technology:

The caption:
Interesting, I think.
If we are going to save what is left to be saved, and restore what can be restored, we are going to need to utilize plutonium intelligently, continuously working to improve processing. It's nice to see this work along those lines.
Another Orwellian "Green is Black" Day in Germany.
Today, as yesterday, Germany has the second worst CO2 intensity for its power grid in Europe.
(I remarked on this yesterday for both the 30 day trend and the daily trend: 30 Day German Carbon Intensity Is 709 g CO2/kWh, Second Highest in Europe After Poland.)
Today's color coded Electricity Map for Europe:

Apparently the wind isn't blowing in Germany (again) and the sun isn't shining brightly at 8:45 in the morning (again).

Whenever the wind isn't blowing and the sun isn't shining, the Germans apparently feel it's their "right" to burn coal and dump the waste directly into the planetary atmosphere driving climate change and killing people.
Fuckers.
Germany is run by a coalition including members of the ignorance purveying antinuke cult "Greenpeace." For truth in advertising purposes perhaps that long lived cult should change its name to "CoalPiece."
History will not forgive us, nor should it.
Omics Analysis of an Organism That Metabolizes Polycyclic Aromatic Hydrocarbons (PAH).
The paper that I'll briefly discuss is this one: Multi-Omic Profiling of a Newly Isolated Oxy-PAH Degrading Specialist from PAH-Contaminated Soil Reveals Bacterial Mechanisms to Mitigate the Risk Posed by Polar Transformation Products, Sara N. Jiménez-Volkerink, Joaquim Vila, Maria Jordán, Cristina Minguillón, Hauke Smidt, and Magdalena Grifoll Environmental Science & Technology 2023 57 (1), 139-149.
PAH's are components of dangerous fossil fuel waste that are found in air, water, and land both from combustion and spills, leaks and mining (including drilling) residues. The mechanism by which they work as carcinogens includes the fact that they are planar molecules which can insert into the major and minor grooves of DNA. If partially oxidized they can form adducts with nucleosides, inducing a metabolic cascade leading to uncontrolled cell division. There are many pathways by which PAHs can oxidize, and the problem is to further break down these molecules before they can reach carcinogenic concentrations.
I generally think of them as being recalcitrant to bioremediation, as they are toxic, and so I was pleased to see this "Omics" paper showing the metabolic pathway by which an organism, Sphingobium sp. AntQ-1, that is found in soils oxidizes PAH's in order to generate cellular energy. "Omics" includes proteomics, the structure and function of proteins, lipidomics, the structure and function of lipids, genomics, the structure and function of DNA, RNA and related compounds, and finally glycomics, the structure and function of sugars.
The paper is a detailed analysis of the proteome and the coding genes, as well as the pathway of degradation.
From the introduction to the paper:
Bioremediation is the most sustainable technology for the clean-up of hydrocarbon contaminated sites due to its cost-effectiveness, low environmental footprint, and capability to restore key natural soil functions. (7) Nevertheless, some studies have reported an eventual increase in genotoxicity in bioremediated PAH-polluted soils that has been associated to polar fractions enriched in oxy-PAHs resulting from PAH biotransformations. (8,9) A recent study has identified 2H-naphtho[2,1,8-def]chromen-2-one, a bacterial metabolite of pyrene, as a main contributor of the genotoxicity observed in PAH-polluted soil after treatment in an aerobic bioreactor. (9) Due to their physicochemical properties, oxy-PAHs have greater bioavailability and environmental mobility than PAHs, (10,11) and a number of them have been demonstrated to present higher (geno)toxic, mutagenic, and carcinogenic activities than their unsubstituted counterparts. (12,13) Several bacterial isolates are known to produce oxy-PAHs as dead-end products during aerobic metabolism of PAHs. (14,15) Further biodegradation of such oxy-PAHs has been reported after stimulation of the microbial activity in soils; (3,4) however, little is known about the microorganisms and mechanisms underlying their fate in the environment.
9,10-Anthraquinone (ANTQ), the ready oxidation product from anthracene (ANT), is one of the most commonly found oxy-PAHs in PAH-contaminated soils (1,10) and has been classified as possibly carcinogenic to humans (group 2B) by the International Agency for Research on Cancer. (16) ANTQ has been reported as a dead-end transformation product of anthracene by pyrene-degrading mycobacteria, (14,17) and its formation and eventual removal during biological treatment of contaminated soils has been reported. (3,18,19) In a PAH-contaminated soil from a former manufactured-gas plant, ANTQ biodegradation was associated to uncultured Sphingomonas and Phenylobacterium species (20) detected by DNA stable-isotope probing, but the specific metabolic mechanisms involved remain to be elucidated.
In this study, we report the isolation of a bacterial strain (AntQ-1) able to utilize 9,10-anthraquinone as a sole carbon source from PAH-contaminated soil from a historical wood-treating facility in the south of Spain. This isolate is used as a model to shed light on the microbial mechanisms driving oxy-PAH biodegradation in contaminated soils...
Regrettably I won't have much time to go into the paper's details, but a few graphics can evoke the general nature.
The first evokes the genome and translational proteome:

The caption:
The metabolic pathway leading to the complete breakdown of the oxidized PAHs.

The caption:
In deliberately contaminated test soils in the laboratory, the organism was shown to thrive and the concentration of the dangerous pollutants declined significantly.
Very cool, I think.
Have a nice day tomorrow.
30 Day German Carbon Intensity Is 709 g CO2/kWh, Second Highest in Europe After Poland.

Coal dependent Poland is at 934 g CO2/kwh. To address this terrible record, Poland is planning to go nuclear against climate change. They at least have a real plan to get better.
Electricity map, Germany (Accessed 1/25/23, 3:45 EST US)
As of this writing, 9:35 Berlin time, 01/25/23, German carbon intensity is 734 g CO2/kwh, again the second worst in Europe.

Their much heralded wind capacity of 66.6 GW is actually producing 3.62 GW of power, for a capacity utilization of 5.49%. It is, in effect, nearly useless, and there is nothing that Germans can do to make the wind blow when their demand is rising.
In Germany "Green" is black.
In case you ever thought antinukes give a rat's ass about climate change, they don't.
Recovery of indium, yttrium, neodymium, and lanthanum by urban mining via pseudoprotic ionic...
...liquids.
The paper to which I'll briefly refer to in this post is this one: Pseudo-Protic Ionic Liquids for the Extraction of Metals Relevant for Urban Mining, Claudia Castillo-Ramírez and Camiel H. C. Janssen, Industrial & Engineering Chemistry Research 2023 62 (1), 627-636.
In my generation we were trained to believe that recycling was a kind of magic. Many of us dutifully separated our plastic, our glass, and our aluminum for the purpose or recycling or, more realistically, for the purpose of letting ourselves believe we were green. Before the rise of diesel powered garbage trucks for municipal recycling, I personally used to load all this crap up into the trunk of my car and drive it to a recycling center, whereupon I would be offered a nominal sum for my efforts.
In the case of plastic, the results of our efforts are in. There are almost no living things, including humans, that do not contain micro or nano plastics in their flesh; there seems to be no upper limit to how much. It is now understood that not only are all of the oceans and lakes laced with plastics, but apparently we are breathing nano plastics suspended in air.
Here's a nice paper on the topic: Bioeffects of Inhaled Nanoplastics on Neurons and Alteration of Animal Behaviors through Deposition in the Brain Xiaoyan Liu, Yingcan Zhao, Jiabin Dou, Qinghong Hou, Jinxiong Cheng, and Xingyu Jiang Nano Letters 2022 22 (3), 1091-1099
Um...brains...you don't say? I wonder if inhaling too much plastic accounts for Republicans and anti-nukes.
Speaking of anti-nukes, there's a fun one around here who likes to call up a link to the USGS reference on indium in hopes of embarrassing me for my contention that the supply of indium is limited, and that there probably isn't plenty of indium to make billions and billions of CIGS type solar cells. This is the sort of moron who believes that we should drive the nuclear industry out of business by mining the shit out of the Earth, roasting zinc sulfide ores from which indium is obtained (presumably with forests as a fuel source), copiously releasing sulfur oxides in the process, this because dumb people are terrified of traces of radiation even as they don't give a rat's ass about the millions of people who die each year from air pollution while we all wait increasingly breathlessly for the grand solar and wind nirvana that did not come, is not here, and won't come.
After nearly 3/4 of a century of cheering the solar industry, at an expense of trillions of dollars, in 2021 the solar industry produced just 5 Exajoules of the 624 Exajoules humanity consumed that year:
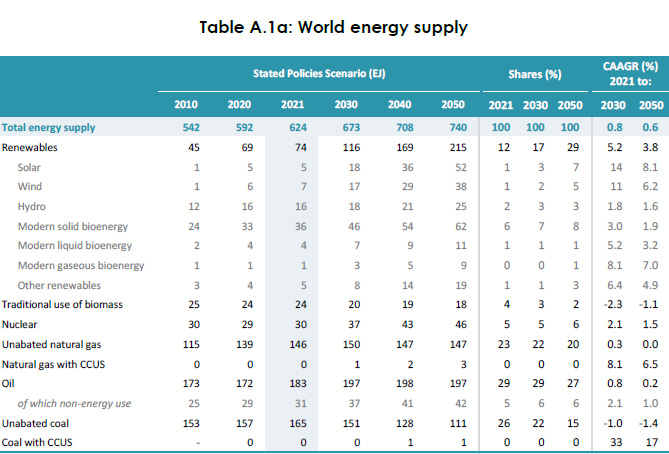
Source: 2022 IEA World Energy Outlook Table A 1a, page 435.
Don't worry. Be happy. I'm sure we'll be able to increase the solar industry's supply of indium even if we need to tear the shit out of the ocean floor to do so, and increase the solar output to 624/5 * 100 = 12,480%. And we'll make lots of batteries too, because the supply of cobalt slaves to dig cobalt for our Powerwalls® in our "green" (albeit deforested) nirvana.
Perhaps the person with access to the USGS Indium link should write the authors of the paper cited at the outset. They seem to have not gotten the message that "solar will save us" antinuke wants to offer us, that indium is "no problem." Apparently people dying from extreme heat while we wait for the solar nirvana are no problem either.
One hears these sorts of things and one doesn't want to believe it.
The authors of the paper writes about indium, thus:
By the way, the existence of an "energy transition," which shows up all over the place, even in scientific papers, is a nonsense statement given the data from the IEA World Energy Outlook produced above. The use of coal, oil and gas is increasing faster than the solar and wind energy that has become an object of religious worship. Apollo and Aeolus are back, but almost certainly as meaningful in practical terms as they were to citizens of classical Greece, people whose civilization vanished.
The other elements discussed are yttrium, neodymium and lanthanum.
The authors write of lanthanum, neodymium, and yttrium:
Neodymium is widely used in (strong, permanent) magnets. (1) These neodymium magnets are popular for their use in electronic equipment such as microphones and headphones. (1,36) Neodymium can also be found in the magnets used in electric motors. (1,5) The current transition to electric cars, particularly in Europe will only increase future demand. Research has shown that neodymium can be recycled for use in new magnets. (3)
Yttrium saw extensive use in the manufacturing of cathode ray tube (CRT) TVs. (1) Yttrium can be used in superconductors because it becomes superconducting at a temperature superior to the boiling point of nitrogen. Yttrium is considered to be one of the materials with serious shortage in the near future...
The ionic liquids the authors propose as an extraction agent for urban mining (based on dissolution of waste electronics in nitric acid) have the following structure:
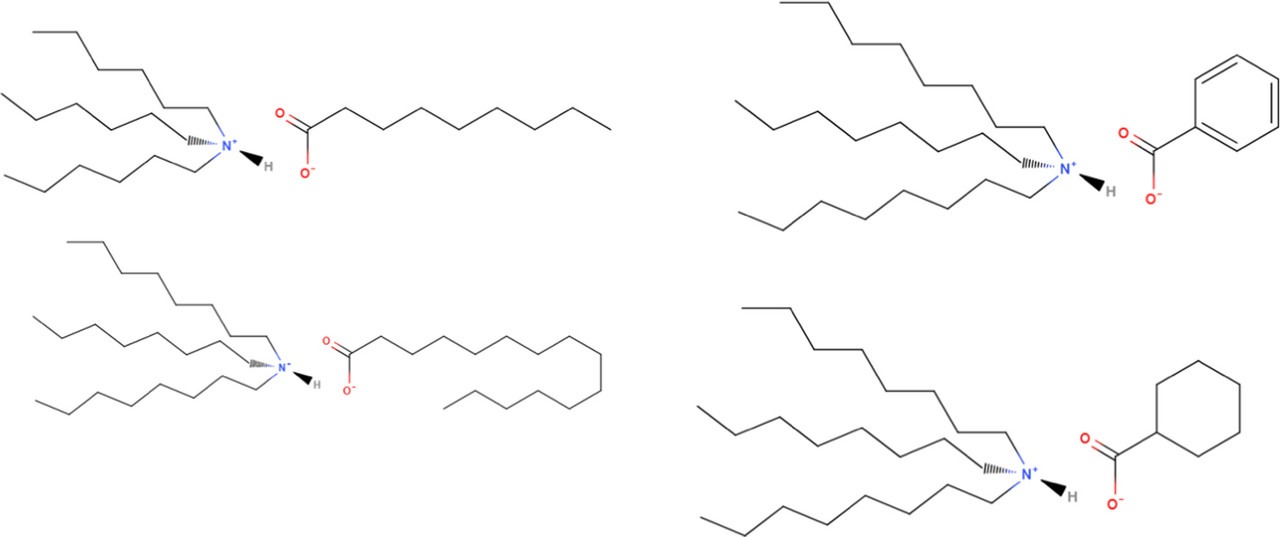
The caption:
The extraction of indium from nitric acid solutions is nearly quantitative:
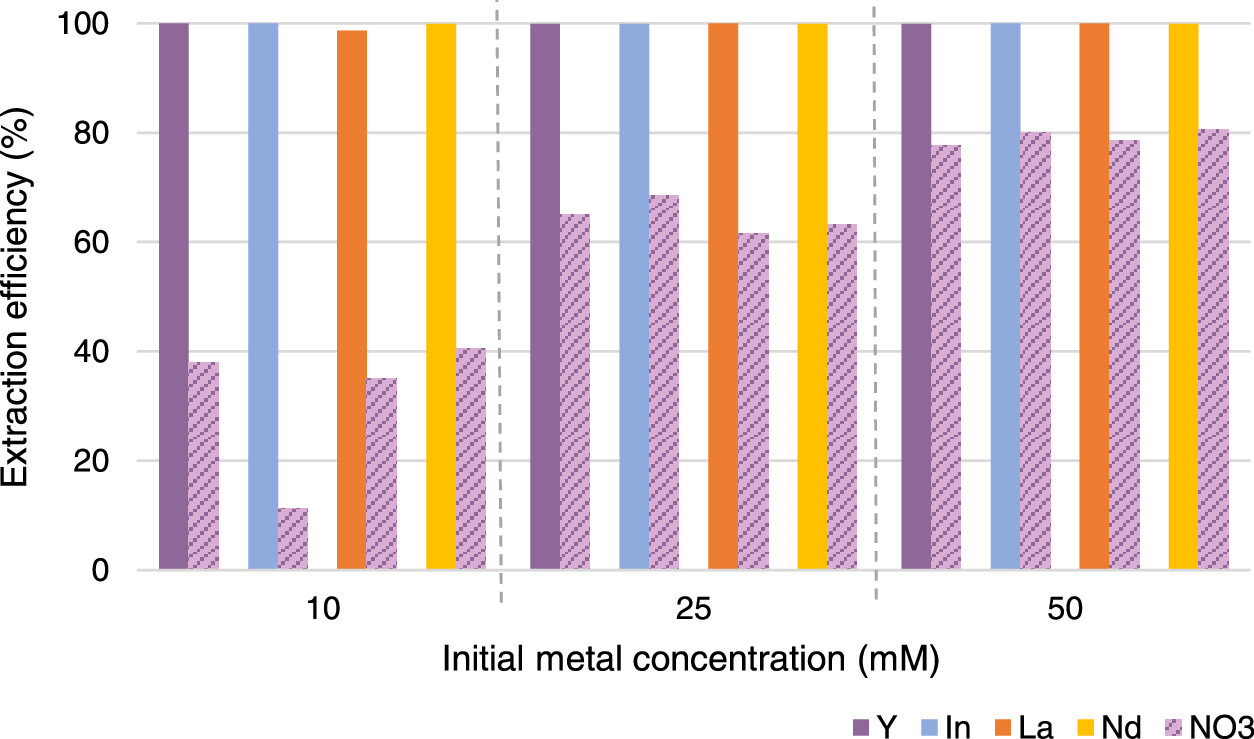
The caption:
However getting the indium out of the extractant is more difficult than getting it in:
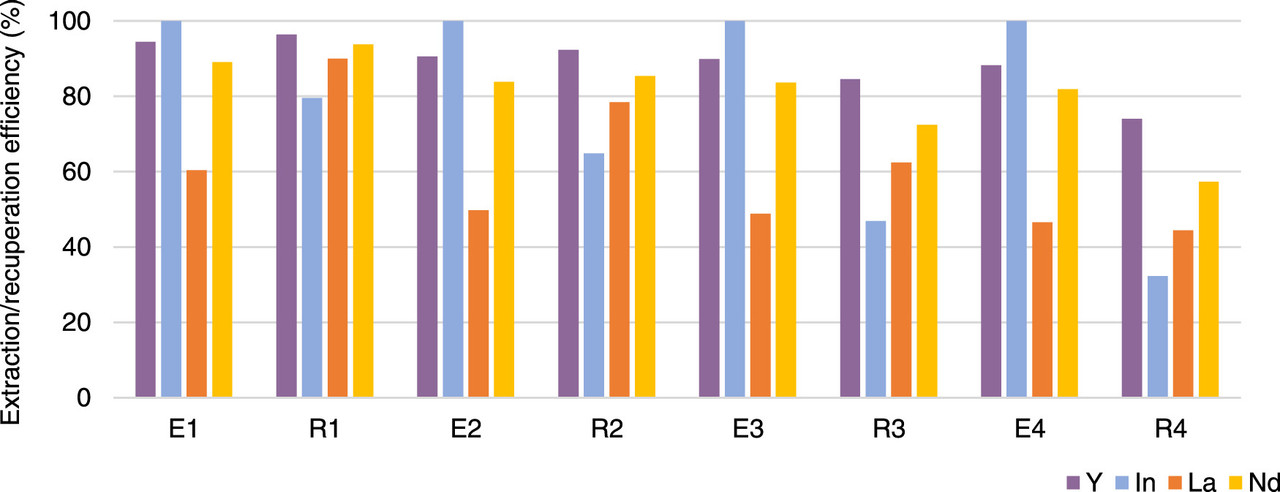
(These last two graphics are representative of the general trends for all the PPIL (Pseudoprotic ionic liquids) discussed in the paper. I have arbitrarily chosen two of these graphics.)
The problem of the baby boomer generation's - my demographic - belief that recycling, currently referred to as "closed cycles" in the literature, will solve things is fantastic, where here the word "fantastic" refers to fantasy and not fabulousness. The issue is tied to transport, and the driver of transport, energy, as well as heat energy. No, you will not be able to get this heat by bullshitting endlessly about the wonders of solar and wind power.
(I find my generation embarrassing.)
No mass cycle can be entirely closed; there will always be process losses, and the word for these losses is "pollution," because the losses are rejected to the environment.
This said, being a baby boomer, I do believe that closed cycles are a worthy aspiration, to the extent possible. However this can only be accomplished with low environmental impact with continuous flow processes, and driving cans and glass and plastic to a recycling center is counter-productive. Further the more dilute a source is, for example indium from a touch screen or CIGS cell, the more energy is required to recover it and the greater the environmental impact. The entropy of mixing is a big, big, big, big deal to overcome, hardly trivial. Thus we need clean and sustainable energy, of which there is only one kind, nuclear energy.
You can imagine that all these solar cells that will become electronic waste just as today's toddlers are graduating from college will be magically recycled, or you can get serious.
It's a choice.
Have a nice day tomorrow.
I'd like to propose another headline for a Harper's article posted on the cover.
It's not like I read the article. I really don't need to see what Francis Fukuyama (one of the authors) thinks about anything any more than I need to understand the plot of the latest Disney movie, whatever it might be.
However, I'll suggest replacing the headline, "Is liberalism worth saving?" with "Is insanity worth embracing?"
The cover:

Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,574