NNadir
NNadir's JournalHow Scary Is Radiation?
A balanced perspective from important nuclear advocates, the Great Chris Keefer and the equally Great Nick Touran:
I'm not generally a big fan of "CleanTechnia," a "renewables will save us" website, but I came across this:
Trump’s DOE Swings At Clean Energy & Accidentally Hits A BullseyeThe excerpt I liked is this one, with which I absolutely agree:
I added the bold. It's wonderful to hear evocation of the 2nd law of thermodynamics, which should immediately and unambiguously kick the hydrogen fantasy to the contents of comic books.
President Biden is a great man, but even great men make bad choices, and support for hydrogen "energy" was such a choice. Hydrogen is not, in fact, primary energy and thus it is a scheme that somehow manages to generate inappropriate enthusiasm that is, in the end, like so called "renewable energy" itself, ultimately, ill advised at best, highly destructive at worst.
Conversely, even bad people can, as the author notes, do good things. The fact that Hitler pushed for the development of the Volkswagen bug, which was for decades one of the most fuel efficient cars on the market, did not make the Volkswagen bug a bad thing.
ICK!!! Going through some old literature I came across a process with an obscenity as a name.
In my general scientific reading, I came across this paper: Adsorption and Chromatographic Behavior of Dispersed Sodium Bismuthate Systems for the Separation of Americium from Curium Samantha A. Labb and Ralf Sudowe Industrial & Engineering Chemistry Research 2025 64 (8), 4516-4524
It caught my eye since I believe that the recovery of the transplutonium actinides is a key technology if we are to save the world, and I'm particularly interested in americium as a potential nuclear fuel owing to the high neutron multiplicity in fission.
I discussed this recently at DU: Some Aspects of the Use of Americium as a Nuclear Fuel There are, as I noted in the post, some technical problems with this idea, but they are, in my view, surmountable.
Invariably, the use of americium as a neat fuel, or even as a mixed fuel, will lead to the generation of curium, in particular the highly radioactive (and valuable) isotope 244Cm. The paper cited above has this to say about americium and curium and their relative position:
The separation of americium (Am) from curium (Cm) has prevailed as one of the most difficult separations to achieve in radioanalytical chemistry, and it is limited by the similar chemical properties of these two elements (e.g., predominate trivalent oxidation states in aqueous acidic media, similar ionic radii). A primary motivation for the development of a rapid and highly selective separation of the two adjacent actinides is the facilitation of a closed nuclear fuel cycle. With no long-term disposal plan for the large inventory of used nuclear fuel (UNF), the implementation of partitioning and transmutation (P&T) is a rational approach toward recycling UNF to increase resource utilization and fuel cycle sustainability. Am and Cm are major contributors to the long-term radiotoxicity of UNF, and their elimination from the waste stream for reuse in a nuclear reactor is necessary. (1,2) Am is generally considered the prime candidate for transmutation, and while Cm transmutation is possible, the high heat load and intense neutron emissions make the use of Cm targets unfavorable. (3,4) Thus, the separation of Am and Cm is required as the final stage of any complete UNF reprocessing strategy. Additionally, the purification of these radionuclides is necessary for target development used in isotope production and cross-section measurements as well as for the analysis of environmental samples...
244Cm produces a power output just shy of 3 watts/gram, which makes it, to my mind, a potential fuel for thermoelectric devices to provide continuous small uninterruptable power sources. A drawback is that it and its decay product, 240Pu release a lot of neutrons from spontaneous fission, although this may not be a drawback is the resultant neutrons are put to good use. The neutron flux from 244Cm is 1.01 X 107 neutrons per gram per second. (cf. M. Salvatores, G. Palmiotti, Radioactive waste partitioning and transmutation within advanced fuel cycles: Achievements and challenges, Progress in Particle and Nuclear Physics, Volume 66, Issue 1, 2011, Pages 144-166). The neutron flux from 240Pu is 1060 neutrons per gram per second. (Cf. Yoshiki Kimura, Masaki Saito, Hiroshi Sagara, Improvement of evaluation methodology of plutonium for intrinsic feature of proliferation resistance based on its isotopic barrier, Annals of Nuclear Energy, Volume 40, Issue 1, 2012, Pages 130-140.)
Anyway.
The paper cited at the top of this post is essentially a chromatographic method relying on oxidized bismuth to oxidize americium to one of its higher, but generally unstable oxidation states.
To me, industrial processes relying on chromatography, although they are known, are less than ideal.
I'm not a solvent extraction (i.e Purex, Truex, etc., etc.) kind of guy. I like fluoride volatility methods. However neither americium nor curium produce very volatile fluorides, but they, along with some lanthanide fission products would be a kind of residue after removal of the volatile fluorides, many of the transition element fission products as well as uranium, neptunium, and plutonium hexafluorides, all of which are volatile. The americium, curium and lanthanide residue would be in the form of fluorides
So I poked around a bit to see if there were papers and came across this paper that's now around 30 years old: Electrochemical separation of actinides and fission products in molten salt electrolyte R. L. Gay; L. F. Grantham; S. P. Fusselman; D. L. Grimmett; J. J. Roy AIP Conf. Proc. 346, 639–645 (1995). It kind of fits the bill at what I was trying to get.
And what is the process called in the text of the paper. With some regret, being forced to mutter an obscenity I really don't like to use, although I'm hardly prudish about using "bad words," let me tell you by quoting the introduction:
Tr**p-S?
Ick.
Maybe the process is cool, but if it ever comes up again - it probably won't - it needs a better name.
Based on the interesting premise of the book jacket, I asked my wife I could buy it, and did so.
The excerpt, that caught my eye:
I have so much to read, so little time to read it. I do hope to get to actually read this book; poignant in these times.
The book: Churchill and Orwell, the Fight for Freedom. The author is Thomas E. Ricks.
Sound like anyone you know?
"...public statements and erratic foreign policy greatly antagonized the international community and are considered by many to have substantially contributed to the fall of..."
I'm reading a fascinating history book 1918, War and Peace about the disintegration of Germany's government at the end of the First World War, a war initiated in part because of imperialist aspirations of the "German Empire" for new territory. They had a lot of trouble trying to assemble a government that could manage the surrender to the French, British, and American allies.
(The behavior of the German army in 1914, when the war broke out, anticipated the behavior of the World War II Germans, mass executions of civilians, that sort of thing.)
As I was reading, I went to Wikipedia to learn about Friedrich Ebert, the accidental Chancellor to whom it fell to (ultimately) organize the Weimar Republic. Then I ended up on the Wikipedia page of Wilhelm II, the last German emperor which contained the excerpt above.
This is not going to end well, 111 years after the outbreak of World War I.
After my adventure with apparent hypoglycemia, my Dr. fitted me with a Dexcom7 continuous glucose monitor.
I'm on ozempic; I've lost weight and got my a1c under control. I've been diagnosed with borderline type 2 diabetes. (It killed one of my grandfathers and it is genetic.)
My doctor kept me on other type II diabetes medicines.
I woke up on Thursday extremely dizzy, almost unable to walk, so I worked from home and went to the doctor. She put me through a series of tests, and then suggested I put on a sample Dexcom7 continuous glucose monitor that gives continuous readings to my cell phone.
Using it, I self-diagnosed - I'm sure the doctor would agree - what was going on as hypoglycemia. I discontinued one of the other diabetes medicines I was using pioglitazone. Anyway, I've left the thing on, and will use it until the battery dies.
It's pretty cool though; I have to say I'm fascinated. If you drink something sweet, it shows up as a spike, lasting about a half an hour. You can see how foods affect you. So far, I've only gone out of range for less than 1% of the time, without taking the pioglitazone.
I don't need this device permanently, but I'm enjoying seeing how foods effect me. I might have behaved better if I'd had it earlier.
An Additive Manufacturing Process for Producing Polymeric Neutron Shields for Fission and Fusion Nuclear Reactors.
Here's a pretty cool paper I almost overlooked, which involves an interesting approach to neutron shielding. Although personally, I'm opposed to wasting neutrons, there are situations in which they need to be managed, particularly in relatively small nuclear reactors. Thus I found the following paper interesting:
Identifying Syndiotactic Polypropylene as a Promising Candidate for Polymer Laser Powder Bed Fusion and Neutron Shielding Materials Ying Han, Hao Jiang, Xiulian Chen, Jun Liu, Ruiqian Zhang, Shiping Song, and Yijun Li Industrial & Engineering Chemistry Research 2025 64 (7), 3785-3794.
From the introductory text:
Neutron radiation is a form of ionizing radiation composed of high-speed neutrons featuring strong penetrating power, (4,5) which allow it to pass through various materials and especially, thermal neutrons with energy range between 0.025–1 keV are most likely to impact human health. (6) Hydrogen-rich polymers and their composites, incorporating elements with high neutron absorption cross sections such as boron (B) and cadmium (Cd), can effectively moderate and absorb neutrons and are frequently used as front-end shielding materials for effective neutron shielding. However, the increasing complexity of neutron shielding device structures, driven by the development of microreactor nuclear power sources, demands manufacturing processes that can efficiently adapt to irregular surface geometries. Unfortunately, the traditional processing techniques like compression molding and injection molding suitable for polyethylene, (7,8) epoxy resins, (9) high-temperature resistant polyimides, (10) flexible rubbers, (11) and hydrogen-rich benzoxazines (HRB), (12) are limited to producing conventional plate-shaped components and lack the capability to fabricate products with complex geometries or special shapes. This significantly restricts the flexibility and design adaptability of shielding parts, making it difficult to effectively wrap protective components for nuclear radiation shielding.
Polymer laser powder bed fusion (PBF-LB/P), a highly representative additive manufacturing technology, (13) can precisely and rapidly produce complex structures to meet the protection needs of irregular geometries in microreactor nuclear power sources. However, the primary sintering materials in the PBF-LB/P domain are semicrystalline long-chain polyamides (PA), (14,15) such as polyamide 6 (PA6), polyamide 12 (PA12), and their composites, as well as thermoplastic polyurethane (TPU). Due to their high cost and significant postcondensation phenomena, polyamide powders are not suitable as base materials for large-scale neutron shielding device production. Polypropylene (PP), one of the most cost-effective general-purpose plastics, offers advantages such as low density, low processing temperature, and good chemical resistance. The high hydrogen content in PP molecules enhances its neutron moderation efficiency. However, research on the application of PP in the PBF-LB/P field has been limited, primarily due to the high melting temperature (Tm > 165 °C) of isotactic polypropylene (iPP) and its rapid crystallization rate and high crystallinity, which lead to warping and printing failures during the sintering process, or significant deviations in part accuracy due to postcondensation. Very recently, syndiotactic polypropylene (sPP), with a different spatial configuration from iPP, has –CH3 groups alternately arranged on both sides of the molecular chain, resulting in greater flexibility and enhanced resistance to thermal degradation. (16,17)...
...Based on the crystallization behavior of syndiotactic polypropylene (sPP), this study successfully achieved the PBF-LB/P processing of sPP powder for the first time and applied it to neutron radiation shielding, addressing three key objectives: (1) revealing the isothermal and nonisothermal crystallization behavior of sPP to verify its processability in PBF-LB/P; (2) determining the optimal printing parameters for sPP in PBF-LB/P processing to ensure low shrinkage and dimensional stability; and (3) evaluating the neutron shielding performance of sPP and its products. (24−26) This study not only expands the range of materials suitable for PBF-LB/P but also provides new insights into enhancing the processing methods for complex and precise polypropylene structures...
An interesting graphic, a graphic reflecting combinatorial optimization, from the paper:
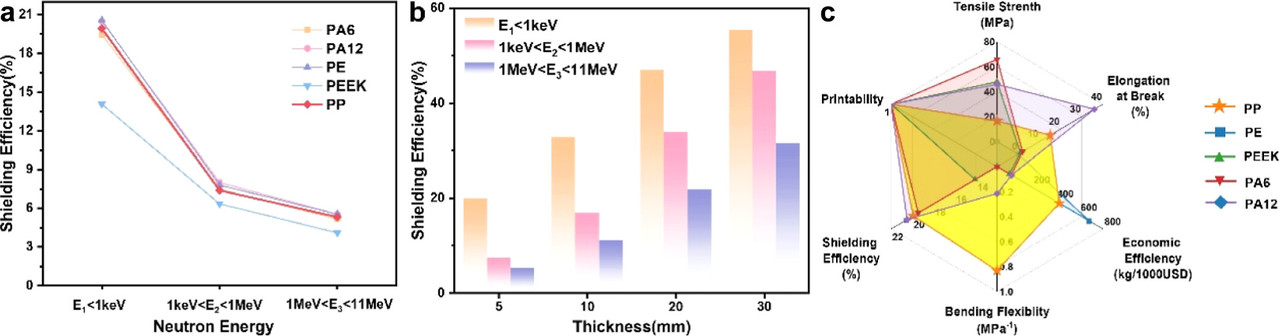
The caption:
My son is seeking his Ph.D. in a field involving additive manufacturing, although his work involves 3D printing of metals as opposed to the more widely applied 3D printing of polymers. I'll share this paper with him.
I trust you're having a pleasant Sunday in spite of the impending collapse of our country by suicide.
Life Cycle Analysis of "Carbon Free" Steel Making.
The paper to which I'll refer in this post is this one: Prospective Life Cycle Assessment Suggests Direct Reduced Iron Is the Most Sustainable Pathway to Net-Zero Steelmaking Arezoo Azimi and Mijndert van der Spek Industrial & Engineering Chemistry Research 2025 64 (7), 3871-3885.
The paper is open sourced, so there's no need to excerpt it too much, although I'll add a few excerpts, since the proposed LCA (Life Cycle Analysis" involves hydrogen, which is not, despite much bullshit that flies around here and elsewhere, carbon free. Hydrogen is made, overwhelmingly by the steam reforming of dangerous fossil fuels. However, in theory, if not in practice, hydrogen could - there is a huge difference between could and is - be made by the only scalable form of primary energy available, nuclear energy.
The bullshit in this LCA paper is based on the fantasy that electrolysis to provide hydrogen using so called "renewable energy" is economically and environmentally available. It isn't. Germany (BASF) shut ammonia plants in the last several years because Putin cut off their supply of dangerous natural gas, which was their major source of hydrogen via the SMR, steam methane reforming, process.
Nevertheless, it appears, from the text, that in Europe, steel plants that do not use the coal based iron reduction, substituting hydrogen, are being built. This is a very bad idea from my perspective, not quite as bad as Germany shutting its nuclear plants, but up there.
From the text:
Decarbonizing the steel industry is crucial due to its significant impact on climate change. The steel industry is highly energy- and emission-intensive, ranking first in CO2 emissions and second in energy consumption among industrial sectors. According to the World Steel Association, (2) in 2021, the iron and steelmaking industry emitted 1.91 tonnes of CO2 per tonne of crude steel cast produced and consumed 20.99 GJ of energy per tonne. It is currently the largest industrial consumer of coal, which provides around 75% of its energy demand and is essential for producing coke, a key component in the chemical reduction of iron ore in blast furnaces.
To meet global climate goals, CO2 emissions from the iron and steel industry must decline toward net-zero by 2050. Strategies for achieving deep emissions reductions in steelmaking include CO2 capture and storage (CCS), fuel switching (e.g., hydrogen or bioenergy), direct electrification, and innovative process designs such as direct reduction of iron ore with hydrogen. Factors such as energy prices, technology costs, and the availability of raw materials will significantly influence the adoption of these technologies. (3) For example, access to low-cost renewable electricity provides a competitive advantage to hydrogen-based steelmaking. Additionally, to achieve 2050 climate goals, the rapid deployment of technologies that are currently in early development stages is required. For example, the International Energy Agency (IEA) estimated that a new hydrogen-based direct reduction plant needs to be deployed every month once the technology is commercially available. (1)
The Western European steel industry is undergoing a significant transition from coal-based production methods to more sustainable power-based processes. This shift is being spearheaded by several countries committed to reducing carbon emissions. In the United Kingdom, a major transformation is underway, with the planned shutdown of two primary steelmaking sites operated by British Steel in Scunthorpe and Tata Steel in Port Talbot. These sites, which currently rely on traditional blast furnace and basic oxygen furnace (BF/BOF) processes, will be replaced by electric arc furnaces (EAFs) that focus on recycling scrap steel (while we note this will remove the UK’s primary steelmaking capability). This move represents a key step in reducing the carbon footprint of the UK’s steel industry. Germany is also making strides toward greener steel production. ArcelorMittal has announced plans to integrate hydrogen-based processes in its steelmaking plants in Hamburg, Bremen, and Eisenhüttenstadt by the mid-2020s. The latter two plants will transition to EAFs as part of this initiative. Additionally, ThyssenKrupp has committed to building a hydrogen-based direct reduced iron (DRI) plant in Duisburg by 2025, further solidifying Germany’s leadership in sustainable steelmaking. (4) In France, the steel industry continues to prioritize emissions reduction through carbon capture, utilization, and storage (CCUS) technologies. The country operates five blast furnaces with a combined production capacity of approximately 15 million tonnes of primary steel annually. While France remains focused on CCUS, (4) Spain is taking significant steps toward decarbonization with ArcelorMittal’s agreement to invest 1 billion euros in its BF/BOF steelmaking plant in Gijón. This investment will fund the development of a green hydrogen DRI unit, complemented by a hybrid EAF, marking a major shift in Spain’s steel production landscape. (5) Sweden’s SSAB (6) is leading a groundbreaking initiative called “Toward Fossil-Free Steel,” in collaboration with industrial and research partners. The project aims to transform Nordic strip production to achieve net-zero emissions by around 2030. As part of this effort, the SSAB plans to convert its Luleå and Raahe sites into mini mills with EAFs and rolling mills in Sweden and Finland, respectively. The company will further develop its Borlänge and Hämeenlinna plants to align with these new production processes. Belgium’s ArcelorMittal Gent (7) is set to electrify and decarbonize its existing blast furnaces by utilizing captured CO2 emissions through a testing pilot carbon capture unit developed by Mitsubishi Heavy Industries (MHI). These emissions will be converted back into carbon monoxide (CO) using plasma technology from D-CRBN. This innovative process will reduce coal usage in blast furnaces and decrease the future need for green hydrogen. In The Netherlands, Tata Steel IJmuiden, the country’s only operating BF/BOF steel facility, is embarking on a significant transformation as part of the “Green Steel Plan.” By 2030, the site’s largest blast furnace, BF7, is planned to be replaced by an EAF, while a new DRI plant will take the place of one of the company’s coke-making facilities. (8)...
If one looks, one can see that the usual bullshit is clear in this paper, notably CCS, carbon capture and storage, which, like so called "renewable energy" and in fact, the recent burst of "hydrogen hype" which blows through like bad weather every decade or so, is an effort to greenwash fossil fuels.
The conversion of CO2 back to CO (carbon monoxide) is mildly interesting, and represents CCU (carbon capture and utilization) as opposed to CCS, (carbon capture and storage) and to my mind might be worthy over the long term of some consideration, particularly if generated in a nuclear Allam cycle coupled to reformation of waste organic matter, but the fact remains that European electricity is not "green" except in France, Sweden, and Norway, the first owing to its nuclear capacity, the second from a combination of electricity and marginally "green" hydroelectricity, and the latter almost purely from hydroelectricity, although Norwegian wealth derives from the export of dangerous natural gas.
As noted elsewhere in the scientific literature with respect to China, the use of hydrogen based processes makes things worse, not better:
Subsidizing Grid-Based Electrolytic Hydrogen Will Increase Greenhouse Gas Emissions in Coal Dominated Power Systems Liqun Peng, Yang Guo, Shangwei Liu, Gang He, and Denise L. Mauzerall Environmental Science & Technology 2024 58 (12), 5187-5195.
This paper is in the "peer reviewed" scientific literature. "Peer review" is sometimes treated as if it renders a paper oracular, which, in fact, it doesn't. When reading anything including but no limited to "peer reviewed" primary scientific literature, critical thinking is required - not often utilized - but at least should be required. The fact that this requirement often vanishes is a reason that the planet is burning.
Hydrogen and CCS are efforts to greenwash fossil fuels. From my perspective, this paper consists of bad ideas and wishful thinking, a key aspect of the latter, being the inclusion of soothsaying, of which sentences containing the phrase "by 2050" (or by such and such year, when the author will mostly likely be dead or in the best case, long retired).
The paper, if you open it and search it has 6 "by 2050" phrases in it. That says everything you need to know.
I trust you're having a nice weekend.
A Highly Political Movie About a Penguin. I thought it was excellent.
My wife and I saw it in our local art theater of which we are members:
The Penguin Lessons
It's set in Argentina at the time of the military fascist takeover in 1976:
An cynical expat English teacher in an Argentine prep school, takes a trip to Uruguay where, trying to hit on a woman, he rescues a penguin from an oil slick to impress her.
He ends up being compelled to adopt the Penguin and is forced to face the moral consequences of living in a fascist state.
It was excellent, and I recommend it highly, drags a bit in the beginning, but hits hard in the end.
It's also timely, as the United States is going fascist.
A Useful Tutorial on the Analysis of Polymers.
Over the years, given the limits of techniques available to me, I have struggled at times, with the characterization of polymers. Since the question only comes up every four or five years or so, I always find myself having to relearn the techniques involved.
Polymer chemistry is, of course, important in a vast number of areas, and, most notably, represents a huge environmental problem in the form of now ubiquitous nanoplastics, now showing up in people's brains (this is actually true) literally. Along with mercury and lead poisoning from the continuing and rising use of dangerous fossil fuels, this may account for the elevation of the stupid and evil by the stupid and evil in the United States and elsewhere.
Since I confess that I sometimes drift back and forth through my journal here to remember what I was considering at some point or another, I thought I'd note the following paper, which is mildly unusual in the primary scientific literature (but certainly not unknown), billing itself as a "tutorial:"
Polymer Molecular Weight Distributions: A Tutorial on Transformations between Number Density, Mass Density, and Linear/Logarithmic Axes Ziqiu Chen, Changhae Andrew Kim, Yi-Yu Wang, Aaron D. Sadow, Anne M. LaPointe, and Baron Peters Industrial & Engineering Chemistry Research 2025 64 (7), 3695-3703.
Some text:
The reactants and products in polymer upcycling can be analyzed by various measurement techniques, such as gas chromatography (GC), gel permeation chromatography (GPC), etc., according to their carbon number and phase. (5) In this process, molecular weight distribution (MWD), also known as molar mass distribution, is often used for polymer characterization. (6−8) The evolution of the MWD over time can be used to provide insights into the polymerization and depolymerization mechanism. (9−11) Polymer MWDs can be represented in several different ways. The simplest representation gives the number or concentration of polymers with lengths between n and n + dn as ρ
The ability to interconvert between MWD representations is essential for drawing accurate conclusions about mechanisms (13) and for comparing results from different experiments on an equal footing. For example, an MWD that appears to be monotonically decreasing in one representation can appear to have a focused maximum in another representation. The alternative representations have been discussed in previous works on polymer MWDs, (14,15) and different MWD representations were widely used in the field of polymer reactions. (16−18)
This paper aims (i) to present general experimental considerations for studying products with a wide range of molecular weights, thermal properties and solubilities, since this is often crucial for comprehensive assessment of depolymerization reactions, (ii) to provide a tutorial on the interpretation of MWDs in different representations, (iii) to collect the conversion formulas between various representations into one article, and (iv) to show how the different representations are used in different experimental and theoretical analyses...
A number of techniques are summarized in the paper; the only one to which I've ever had access is GPC/RI (gel permeation HPLC using a refractive index detector), although I did try to convince a guy in our lab to utilize a mass spec technique with which he had only limited success, and at another time, we once quantified a PEG polymer in blood by LC/MS/MS utilizing the monomer generated by ESI (electrospray ionization, a common mass spec technology. This technology is not mentioned in the paper, nor, interestingly DLS, dynamic light scattering except peripherally; which at various times, I tried to convince my management to explore, albeit unsuccessfully.)
There is some very cool mathematics in the paper - many beyond the analytical results on which I have focused in my career - unfortunately I won't have time to discuss at them, but perhaps we can look at the pictures to get some insight to some techniques:
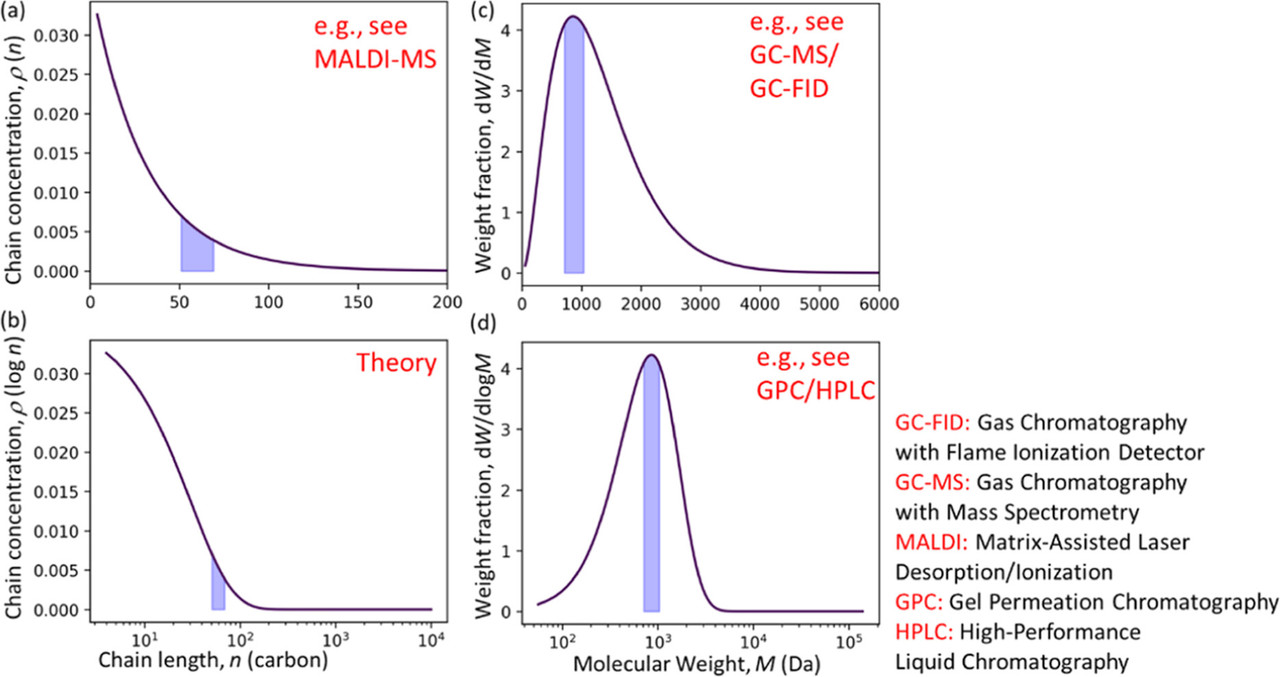
The caption:
A schematic of techniques around expected molecular weight distributions:
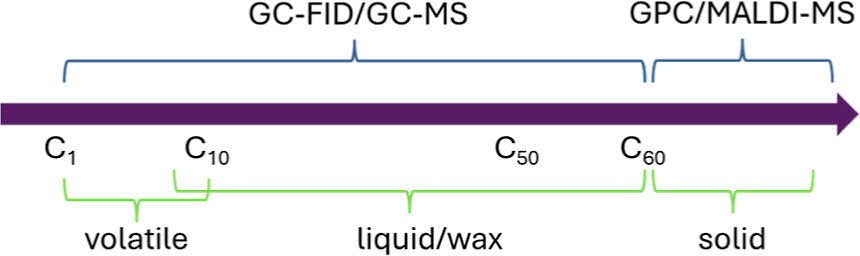
The caption:
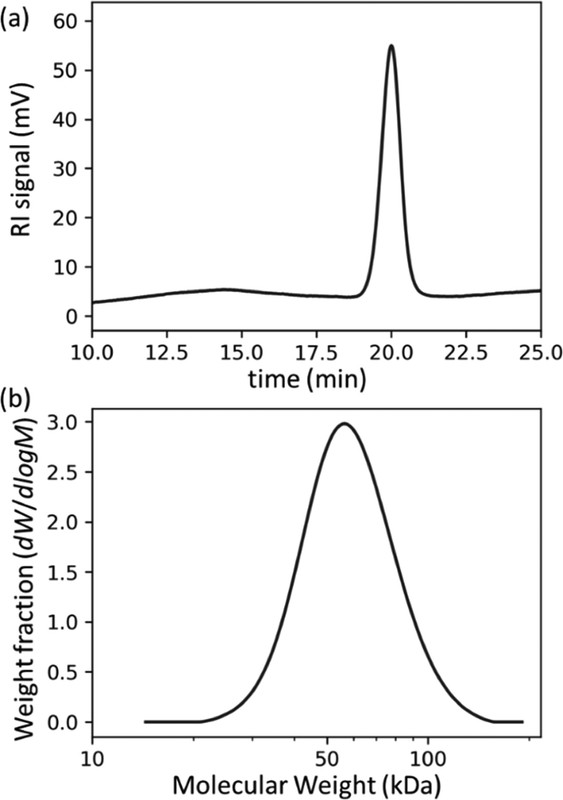
The caption:
This technique is totally new to me; it seems to involve pyrolysis of a polymer on a GC column, which I can't imagine is good for the column, but I probably am missing something:
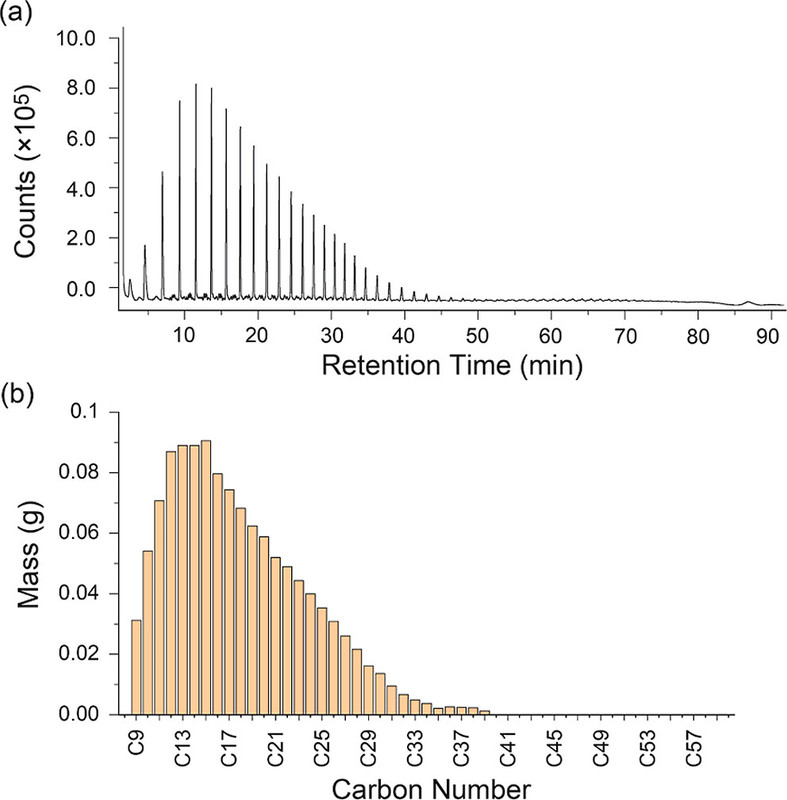
A related technology into which I have looked, without convincing management to buy into an instrument, is DSC, differential scanning calorimetry, which can certainly characterize the types of polymers with which one is dealing, useful in environmental settings where polymer types may be unknown.
Anyway, it's a cool paper. I know there are other chemists on DU - a political website that happily has a nice science section - and perhaps, as I have, they have struggled with polymer characterization - and in any case, given the environmental implications of polymer production and use - it's useful to know.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 35,435